Label The Components Of A Myofibril.
Holbox
Mar 17, 2025 · 7 min read
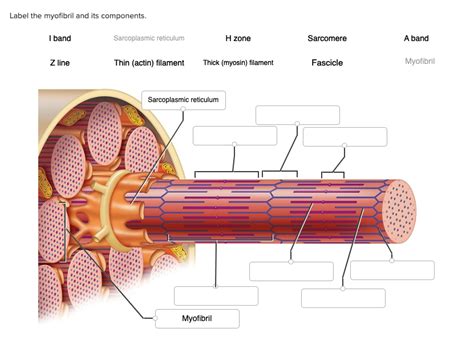
Table of Contents
Labeling the Components of a Myofibril: A Deep Dive into Muscle Structure
Understanding the intricate structure of muscle fibers is crucial for grasping the mechanics of movement and the complexities of muscle contraction. At the heart of this understanding lies the myofibril, a cylindrical organelle packed with the proteins responsible for generating force. This article will provide a comprehensive guide to labeling the components of a myofibril, delving into the structure and function of each element. We'll explore the sarcomere, the basic contractile unit, and its constituent parts, explaining how their arrangement contributes to muscle contraction and relaxation.
The Sarcomere: The Functional Unit of the Myofibril
The myofibril is composed of repeating units called sarcomeres. These are the fundamental units of muscle contraction, arranged end-to-end along the length of the myofibril like beads on a string. The highly organized arrangement of proteins within the sarcomere is what allows for the precise and powerful contractions characteristic of muscle tissue. Let's break down the key components of a sarcomere:
1. Z-lines (Z-discs):
These are the defining boundaries of a sarcomere. They are dense, protein-rich structures that act as anchoring points for the thin filaments (actin). The Z-lines are crucial for maintaining the structural integrity of the sarcomere and for the transmission of force during contraction. Imagine them as the end caps of a spring, holding everything together. Observe the Z-lines closely in electron micrographs – they are essential landmarks for identifying sarcomere boundaries.
2. A-band (Anisotropic band):
This is the darker, central region of the sarcomere. The A-band is characterized by the presence of thick filaments (myosin) and the overlapping portions of thin filaments (actin). The length of the A-band remains relatively constant during muscle contraction because it corresponds to the full length of the thick filaments. Understanding the A-band is key to visualizing the interaction between thick and thin filaments during the contraction cycle.
3. I-band (Isotropic band):
This is the lighter region flanking the A-band. The I-band contains only thin filaments (actin) and extends from the end of the A-band to the next Z-line. The I-band shortens considerably during muscle contraction as the thin filaments slide over the thick filaments. The I-band's change in length is a direct visual indicator of muscle contraction.
4. H-zone (Hensen's zone):
Located in the center of the A-band, the H-zone is the region containing only thick filaments (myosin). During muscle contraction, the H-zone narrows as the thin filaments slide inward, ultimately disappearing at maximal contraction. Observe the H-zone carefully to understand the extent of thin filament overlap with thick filaments.
5. M-line (Midline):
Situated in the middle of the H-zone, the M-line acts as a structural support for the thick filaments. It is composed of proteins that link the thick filaments together, maintaining their alignment and stability. The M-line ensures the precise arrangement of myosin within the sarcomere, contributing to the coordinated contraction of the muscle fibers.
The Protein Players: Actin and Myosin
The sarcomere's functionality relies heavily on the interplay of two key proteins: actin and myosin. These proteins are arranged in a way that facilitates the sliding filament mechanism responsible for muscle contraction.
1. Actin (Thin Filaments):
Actin filaments are composed of polymerized actin monomers arranged in a double helix. Tropomyosin and troponin are associated proteins that regulate the interaction between actin and myosin. Tropomyosin wraps around the actin filament, while troponin consists of three subunits: troponin-T (binds to tropomyosin), troponin-I (inhibits actin-myosin interaction), and troponin-C (binds calcium ions). The regulation of these proteins by calcium is pivotal for muscle contraction.
2. Myosin (Thick Filaments):
Myosin is a motor protein composed of two heavy chains and four light chains. Each heavy chain has a globular head region (myosin head) and a long tail region. The myosin heads possess ATPase activity, meaning they can hydrolyze ATP to generate the energy required for muscle contraction. The arrangement of myosin heads along the thick filament creates the characteristic cross-bridges that interact with actin during contraction. Focus on the myosin heads, as these are the crucial sites for ATP binding and force generation.
The Sliding Filament Mechanism: How Sarcomeres Contract
The contraction of a muscle fiber is a result of the sliding filament mechanism, where the thin filaments slide over the thick filaments, causing the sarcomere to shorten. This process is driven by the interaction between the myosin heads and actin filaments.
-
Calcium Release: A nerve impulse triggers the release of calcium ions (Ca²⁺) from the sarcoplasmic reticulum, a specialized storage organelle within muscle cells.
-
Calcium Binding: The Ca²⁺ binds to troponin-C, causing a conformational change in troponin and tropomyosin. This exposes the myosin-binding sites on actin.
-
Cross-bridge Formation: The myosin heads bind to the exposed actin sites, forming cross-bridges.
-
Power Stroke: ATP hydrolysis provides energy for the myosin heads to pivot, pulling the thin filaments towards the center of the sarcomere (towards the M-line).
-
Cross-bridge Detachment: A new ATP molecule binds to the myosin head, causing it to detach from actin.
-
ATP Hydrolysis and Re-cocking: The ATP is hydrolyzed, and the myosin head returns to its original position, ready to bind to another actin site. This cycle repeats multiple times, resulting in the sliding of thin filaments over thick filaments.
-
Sarcomere Shortening: As the thin filaments slide over the thick filaments, the sarcomere shortens, leading to muscle contraction. The I-band and H-zone shorten while the A-band remains relatively constant in length.
Beyond the Sarcomere: Titin and Nebulin
While actin and myosin are the primary actors in muscle contraction, other proteins play crucial supporting roles in maintaining the structural integrity and functionality of the myofibril. Two important examples are titin and nebulin:
-
Titin: This giant protein spans the entire length of the sarcomere, extending from the Z-line to the M-line. It acts as a molecular spring, providing elasticity and passive tension to the sarcomere. Titin helps to maintain the proper alignment of thick filaments and prevents overstretching of the sarcomere.
-
Nebulin: Associated with thin filaments, nebulin is thought to regulate the length of actin filaments, ensuring uniformity in their length and contributing to the precise arrangement within the sarcomere.
Practical Applications and Further Exploration
Understanding the labeled components of a myofibril has numerous practical applications, including:
-
Diagnosing Muscle Disorders: Knowledge of myofibrillar structure is crucial for understanding and diagnosing various muscle diseases, such as muscular dystrophy, which involve abnormalities in the structure and function of muscle proteins.
-
Developing Therapeutic Strategies: Understanding the mechanisms of muscle contraction is vital for developing therapeutic strategies for muscle injuries, age-related muscle loss (sarcopenia), and other conditions affecting muscle function.
-
Designing Advanced Materials: Researchers are inspired by the structure and function of myofibrils to design biomimetic materials with enhanced mechanical properties, potentially leading to the development of novel biomaterials for tissue engineering and other applications.
This in-depth look at the components of a myofibril provides a strong foundation for further exploration. By understanding the precise arrangement and interactions of proteins within the sarcomere, we can gain a deeper appreciation for the complexity and elegance of muscle contraction, a fundamental process underlying all forms of movement and essential for life itself. Further research into the specifics of protein interactions, regulatory pathways, and the impact of various factors on muscle function will continue to broaden our understanding of this critical biological system. Exploring areas such as the role of calcium channels, the influence of ATP levels, and the impact of various diseases on myofibril structure will yield further insights into this fascinating field. Remember, ongoing research constantly refines our understanding, so stay curious and continue learning!
Latest Posts
Latest Posts
-
A Customer Tells His Current Sales Rep
Mar 17, 2025
-
When Using A Self Managed Team A Manager Should
Mar 17, 2025
-
Match Each Definition To The Level Of Protein Structure
Mar 17, 2025
-
A Fixed Position Production Layout Would Be Particularly Recommended If
Mar 17, 2025
-
When Direct Labor Costs Are Recorded
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Label The Components Of A Myofibril. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
