Indicate Whether Or Not The Following Molecules Are Chiral.
Holbox
Mar 21, 2025 · 6 min read
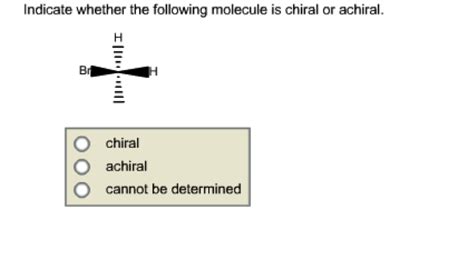
Table of Contents
- Indicate Whether Or Not The Following Molecules Are Chiral.
- Table of Contents
- Chirality: Determining if Molecules are Chiral
- Understanding the Criteria for Chirality
- Analyzing Molecules for Chirality: A Step-by-Step Guide
- Case Studies: Determining Chirality of Various Molecules
- The Importance of Chirality in Various Fields
- Conclusion: A Powerful Tool for Molecular Understanding
- Latest Posts
- Latest Posts
- Related Post
Chirality: Determining if Molecules are Chiral
Chirality, a fundamental concept in organic chemistry and stereochemistry, refers to the handedness of molecules. A chiral molecule is a molecule that is non-superimposable on its mirror image. Think of your hands – they are mirror images of each other, but you cannot overlay one perfectly onto the other. Similarly, chiral molecules exist as two non-superimposable mirror images, called enantiomers. This seemingly simple concept has profound implications in fields ranging from pharmaceuticals to materials science. This article will explore the criteria for chirality and analyze various molecules to determine whether they are chiral or achiral.
Understanding the Criteria for Chirality
Several factors contribute to a molecule's chirality. The most common is the presence of a chiral center, also known as a stereocenter or asymmetric carbon. A chiral center is a carbon atom bonded to four different groups. This tetrahedral arrangement creates the non-superimposability characteristic of chiral molecules.
However, chirality isn't solely dependent on chiral centers. Molecules without chiral centers can still exhibit chirality due to other structural features, such as axial chirality (present in allenes and biphenyls) and planar chirality (found in certain cyclic systems).
Key terms to remember:
- Achiral: A molecule that is superimposable on its mirror image.
- Chiral Center: A carbon atom bonded to four different groups.
- Enantiomers: A pair of non-superimposable mirror image isomers.
- Diastereomers: Stereoisomers that are not mirror images of each other.
- Meso Compound: An achiral molecule containing chiral centers.
Analyzing Molecules for Chirality: A Step-by-Step Guide
To determine if a molecule is chiral, follow these steps:
- Identify all carbon atoms.
- Examine each carbon atom for four different substituents. If a carbon atom has four different substituents, it's a chiral center.
- Consider the overall symmetry of the molecule. Even with chiral centers, a molecule might be achiral due to internal symmetry (e.g., meso compounds).
- Draw the mirror image. If the mirror image is non-superimposable on the original molecule, the molecule is chiral. If they are superimposable, the molecule is achiral.
- Look for other sources of chirality: Axial and planar chirality.
Case Studies: Determining Chirality of Various Molecules
Let's analyze several molecules to illustrate the principles of chirality determination. We'll break down the process for each molecule, highlighting the key features that contribute to its chirality (or lack thereof).
1. 2-Bromobutane:
This molecule has a chiral center at the second carbon atom. The four substituents attached to this carbon are a bromine atom, a methyl group, an ethyl group, and a hydrogen atom. These are all different, making 2-bromobutane chiral. It exists as a pair of enantiomers.
2. 2-Chloropropane:
The second carbon atom in 2-chloropropane has three methyl groups and one chlorine atom. This violates the condition for a chiral center because two substituents are the same (methyl). Therefore, 2-chloropropane is achiral.
3. 1,2-Dibromopropane:
This molecule possesses a chiral center at the second carbon. The four substituents attached to this carbon are a bromine atom, a methyl group, a methylene group (-CH₂-), and a hydrogen atom. All four substituents are different; hence, 1,2-dibromopropane is chiral.
4. 1,3-Dibromopropane:
In 1,3-dibromopropane, there is no carbon atom with four different substituents. The molecule possesses a plane of symmetry that cuts it into two identical halves. Therefore, 1,3-dibromopropane is achiral.
5. Tartaric Acid:
Tartaric acid is a more complex example. It contains two chiral centers. However, the molecule exists in three forms: two enantiomers (d and l-tartaric acid) and a meso compound (meso-tartaric acid). The meso-tartaric acid, despite having two chiral centers, is achiral due to an internal plane of symmetry. This internal plane of symmetry allows for the superimposition of its mirror image. The d and l-tartaric acids are chiral.
6. 1,1-Dibromopropane:
The central carbon in 1,1-dibromopropane is attached to two bromine atoms, a methyl group and a hydrogen. Since it has two identical bromine atoms, the molecule is achiral.
7. 2,3-Dibromobutane:
This molecule displays two chiral centers. Depending on the configuration around these chiral centers, this molecule can be chiral (in the case of two enantiomers and two diastereomers) or achiral (in the case of a meso compound). A detailed analysis of each stereoisomer is necessary to determine the chirality of each specific form.
8. Allenes:
Allenes are a type of molecule that can exhibit axial chirality. The central carbon atoms are bonded to each other in a linear fashion. The substituents on these carbon atoms are arranged in such a way that the molecule lacks a plane of symmetry. Therefore, many allenes are chiral.
9. Biphenyls:
Certain biphenyl compounds can also display axial chirality due to hindered rotation around the central carbon-carbon bond. The large substituents on the phenyl rings prevent free rotation. The different arrangements of these substituents lead to non-superimposable mirror images and thus chirality.
10. Cycloalkanes:
Some substituted cycloalkanes can be chiral if they lack symmetry. For example, 1,2-dimethylcyclopropane is chiral, while 1,1-dimethylcyclopropane is achiral due to a plane of symmetry. The presence or absence of a plane of symmetry determines chirality in these cases.
The Importance of Chirality in Various Fields
The concept of chirality is not just a theoretical exercise; it has significant practical applications in various fields:
-
Pharmaceuticals: Many drugs are chiral molecules. Often, only one enantiomer of a chiral drug is pharmacologically active, while the other may be inactive or even toxic. Understanding and separating enantiomers is crucial for drug development and safety.
-
Food Science: Chiral molecules play a vital role in the flavor and aroma of food. Enantiomers can have different tastes and smells.
-
Materials Science: Chirality affects the physical and chemical properties of materials. Chiral materials can exhibit unique optical, electrical, and mechanical properties.
-
Agriculture: Pheromones, used for insect control, are often chiral molecules. The different enantiomers can have different effects on the target insects.
Conclusion: A Powerful Tool for Molecular Understanding
Determining whether a molecule is chiral or achiral is a fundamental skill in organic chemistry and related fields. By understanding the criteria for chirality, including the presence of chiral centers, axial chirality, and planar chirality, and by carefully analyzing the molecule's symmetry, we can accurately predict and understand its stereochemical properties. This knowledge is essential for advancing research and development across various disciplines. The detailed analysis presented in this article serves as a comprehensive guide to mastering chirality determination, ultimately leading to a deeper understanding of molecular structures and their functions. Continued exploration of chirality will undoubtedly unlock further advancements in fields like medicine, materials science, and beyond.
Latest Posts
Latest Posts
-
Three Capacitors Are Connected As Shown
Mar 28, 2025
-
What Is One Benefit Of Holding Regular System Demos
Mar 28, 2025
-
An Ion Source Is Producing 6li Ions
Mar 28, 2025
-
The Stated Purposed Of Nafta And Usmca Is To
Mar 28, 2025
-
The Central Focus In Hospitality Is
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Indicate Whether Or Not The Following Molecules Are Chiral. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
