Identify Whether Each Monosaccharide Is An Aldose Or A Ketose
Holbox
Mar 30, 2025 · 5 min read
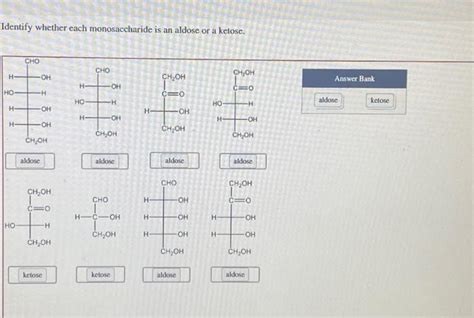
Table of Contents
- Identify Whether Each Monosaccharide Is An Aldose Or A Ketose
- Table of Contents
- Identifying Monosaccharides: Aldoses vs. Ketoses
- Understanding the Carbonyl Group: The Defining Feature
- Aldoses: The Aldehyde Group at the End
- Ketoses: The Ketone Group in the Middle
- Identifying Aldoses and Ketoses: A Practical Approach
- Common Examples of Aldoses and Ketoses
- Important Aldoses:
- Important Ketoses:
- Beyond Simple Classification: Further Considerations
- Stereochemistry: Chirality and Isomerism
- Ring Structures: Cyclization
- Derivatives and Modifications
- The Significance of Aldose/Ketose Classification
- Conclusion: A Cornerstone of Carbohydrate Chemistry
- Latest Posts
- Latest Posts
- Related Post
Identifying Monosaccharides: Aldoses vs. Ketoses
Understanding the fundamental building blocks of carbohydrates is crucial in various fields, from biochemistry and medicine to food science and nutrition. Monosaccharides, the simplest form of carbohydrates, are classified into two major groups based on the position of their carbonyl group: aldoses and ketoses. This comprehensive guide will delve into the intricacies of identifying whether a given monosaccharide is an aldose or a ketose, exploring their structural differences, common examples, and the significance of this classification.
Understanding the Carbonyl Group: The Defining Feature
The key to differentiating aldoses and ketoses lies in the location of the carbonyl group (C=O), a functional group consisting of a carbon atom double-bonded to an oxygen atom. This seemingly small difference in molecular structure leads to significant variations in their chemical properties and biological roles.
Aldoses: The Aldehyde Group at the End
Aldoses possess an aldehyde group (–CHO) at the end of their carbon chain. The aldehyde group is characterized by the carbonyl carbon being bonded to a hydrogen atom and another carbon atom. This structural feature dictates their reactivity and participation in various biochemical pathways.
Ketoses: The Ketone Group in the Middle
Ketoses, on the other hand, have a ketone group (C=O) within their carbon chain. In a ketose, the carbonyl carbon is bonded to two other carbon atoms. This internal position of the carbonyl group distinguishes ketoses from aldoses and influences their chemical behavior.
Identifying Aldoses and Ketoses: A Practical Approach
Identifying whether a monosaccharide is an aldose or a ketose involves a systematic examination of its structure. Here's a step-by-step approach:
-
Identify the Carbonyl Group: Locate the carbonyl group (C=O) within the monosaccharide's structure.
-
Determine its Position: Analyze the position of the carbonyl group relative to the carbon chain.
-
Aldose or Ketose?
- Terminal Carbonyl Group (C=O at the end): The monosaccharide is an aldose.
- Internal Carbonyl Group (C=O within the chain): The monosaccharide is a ketose.
Let's illustrate this with some examples:
Common Examples of Aldoses and Ketoses
Several common monosaccharides serve as excellent examples to solidify our understanding of aldose and ketose identification.
Important Aldoses:
-
Glyceraldehyde (3-carbon aldose): The simplest aldose, glyceraldehyde forms the basis for understanding the stereochemistry of other monosaccharides. It exists as two enantiomers, D-glyceraldehyde and L-glyceraldehyde.
-
Erythrose (4-carbon aldose): A four-carbon aldose with potential applications in various chemical synthesis.
-
Ribose (5-carbon aldose): A crucial component of RNA (ribonucleic acid) and several coenzymes. Ribose's structure is essential for the function of genetic material.
-
Arabinose (5-carbon aldose): Found in various plant gums and hemicelluloses.
-
Xylose (5-carbon aldose): Commonly found in wood and agricultural byproducts.
-
Glucose (6-carbon aldose): The most abundant monosaccharide, a primary energy source for living organisms. Glucose plays a vital role in metabolism.
-
Galactose (6-carbon aldose): A component of lactose (milk sugar) and glycolipids. Galactose is crucial for brain development.
-
Mannose (6-carbon aldose): Present in various polysaccharides and glycoproteins.
Important Ketoses:
-
Dihydroxyacetone (3-carbon ketose): The simplest ketose, often used as a moisturizer in cosmetics.
-
Fructose (6-carbon ketose): Found in fruits and honey, fructose is known for its sweetness. It's also a significant component of sucrose (table sugar).
-
Sorbose (6-carbon ketose): Used in the production of vitamin C (ascorbic acid).
-
Tagatose (6-carbon ketose): A rare sugar with potential health benefits, often used as a low-calorie sweetener.
Beyond Simple Classification: Further Considerations
While the simple classification into aldoses and ketoses is fundamental, understanding additional structural features enhances our understanding of monosaccharide properties.
Stereochemistry: Chirality and Isomerism
Many monosaccharides exhibit chirality, meaning they possess chiral centers (carbon atoms bonded to four different groups). This leads to the existence of stereoisomers—molecules with the same chemical formula but different spatial arrangements. D- and L- isomers are common examples of this, differentiating based on the orientation of the hydroxyl group on the chiral carbon furthest from the carbonyl group.
Ring Structures: Cyclization
In aqueous solutions, monosaccharides typically exist in cyclic forms, forming either pyranose (six-membered ring) or furanose (five-membered ring) structures. This ring formation involves an intramolecular reaction between the carbonyl group and a hydroxyl group on the same molecule. Understanding ring structures is critical for comprehending the interactions of monosaccharides in biological systems.
Derivatives and Modifications
Monosaccharides can undergo various modifications, including the addition of functional groups (e.g., amino groups, phosphates) or the formation of glycosidic linkages with other molecules. These derivatives significantly alter their properties and biological functions.
The Significance of Aldose/Ketose Classification
The classification of monosaccharides as aldoses or ketoses is not merely an academic exercise. It has profound implications for:
-
Metabolic Pathways: The specific metabolic pathways involved in the breakdown and synthesis of monosaccharides are heavily dependent on whether they are aldoses or ketoses. Enzymes have specific binding sites that recognize the structural features associated with each type.
-
Chemical Reactivity: Aldoses and ketoses exhibit distinct reactivity due to the different nature of their carbonyl groups. This influences their interactions with other molecules and their participation in chemical reactions.
-
Biological Functions: The distinct properties of aldoses and ketoses determine their diverse roles in various biological processes. For example, glucose (aldose) is a primary energy source, while fructose (ketose) plays a crucial role in fruit metabolism.
-
Industrial Applications: The unique properties of aldoses and ketoses make them valuable raw materials in various industries, including food processing, pharmaceuticals, and cosmetics.
Conclusion: A Cornerstone of Carbohydrate Chemistry
Identifying monosaccharides as aldoses or ketoses is a cornerstone of understanding carbohydrate chemistry and biochemistry. By systematically examining the position of the carbonyl group and appreciating the intricacies of stereochemistry and ring structures, we can gain a deeper appreciation for the diversity and crucial roles these molecules play in biological systems and various industries. Mastering this fundamental classification enables further exploration of more complex carbohydrate structures and their biological significance. The detailed understanding of aldoses and ketoses lays the groundwork for exploring the fascinating world of oligosaccharides and polysaccharides – complex carbohydrate structures built from these basic units. The knowledge gained forms a solid foundation for further studies in glycobiology, a rapidly expanding field focused on the roles of carbohydrates in biology and medicine.
Latest Posts
Latest Posts
-
Label The Reproductive Structures Of The Female Pelvis
Apr 02, 2025
-
Convert The Given Masses From The Derived Units To Grams
Apr 02, 2025
-
Identify The Indentation That Is Inferiorolateral To The Auricular Surface
Apr 02, 2025
-
Overhead May Be Applied Based On
Apr 02, 2025
-
Knowledge Courage Patience And Honesty Are Examples Of
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Identify Whether Each Monosaccharide Is An Aldose Or A Ketose . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
