Identify The Major And Minor Products Of The Following Reaction
Holbox
Mar 21, 2025 · 5 min read
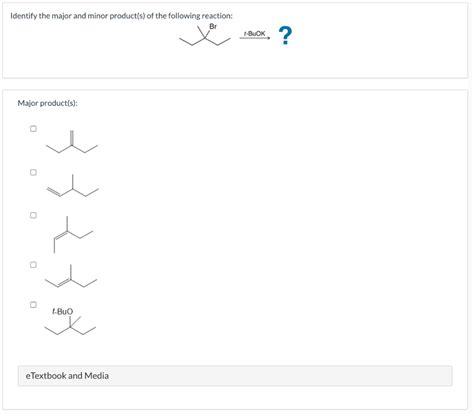
Table of Contents
- Identify The Major And Minor Products Of The Following Reaction
- Table of Contents
- Identifying Major and Minor Products in Chemical Reactions: A Comprehensive Guide
- Understanding Reaction Mechanisms: The Key to Prediction
- Examples of Reaction Mechanisms and Product Prediction:
- Factors Influencing Product Distribution
- 1. Reaction Conditions:
- 2. Steric Hindrance:
- 3. Stability of Intermediates and Products:
- 4. Kinetic vs. Thermodynamic Control:
- 5. Regioselectivity and Stereoselectivity:
- Advanced Techniques for Product Prediction
- Examples of Complex Reactions and Product Analysis
- Conclusion: A Holistic Approach
- Latest Posts
- Latest Posts
- Related Post
Identifying Major and Minor Products in Chemical Reactions: A Comprehensive Guide
Predicting the major and minor products of a chemical reaction is a cornerstone of organic chemistry. It requires a deep understanding of reaction mechanisms, reaction kinetics, and the inherent properties of reactants and reaction conditions. This article delves into the strategies and principles involved in identifying the major and minor products, focusing on various reaction types and influencing factors.
Understanding Reaction Mechanisms: The Key to Prediction
The foundation of predicting reaction outcomes lies in understanding the reaction mechanism. A reaction mechanism is a step-by-step description of how reactants transform into products. This involves identifying intermediate species, transition states, and the rate-determining step. The rate-determining step is the slowest step in the mechanism, and it dictates the overall reaction rate. Understanding this step is crucial, as it often determines the major product formed.
Examples of Reaction Mechanisms and Product Prediction:
-
SN1 Reactions: In unimolecular nucleophilic substitution (SN1) reactions, the rate-determining step involves the formation of a carbocation intermediate. The more stable the carbocation, the faster it forms, leading to a higher yield of the product derived from it. Therefore, tertiary carbocations lead to the major product, while primary carbocations form minor products due to their instability. Rearrangements can also occur, leading to unexpected products.
-
SN2 Reactions: In bimolecular nucleophilic substitution (SN2) reactions, the reaction proceeds through a single concerted step. Steric hindrance plays a significant role; bulky substrates react more slowly, leading to lower yields. Therefore, less hindered substrates favor SN2 reactions and produce the major product. The stereochemistry is also inverted in SN2 reactions.
-
E1 and E2 Reactions: Elimination reactions (E1 and E2) compete with SN1 and SN2 reactions, respectively. E1 reactions proceed via a carbocation intermediate, similar to SN1 reactions. The stability of the carbocation again dictates the major product; more substituted alkenes are generally favored (Zaitsev's rule). E2 reactions are concerted, and the stereochemistry influences product formation; anti-periplanar geometry is preferred. The Zaitsev product (more substituted alkene) is usually the major product.
-
Addition Reactions: In addition reactions, the reactants add across a double or triple bond. Markovnikov's rule often dictates the regioselectivity of addition reactions to unsymmetrical alkenes. The electrophile adds to the carbon atom with more hydrogen atoms, leading to the major product. Anti-Markovnikov addition can occur in the presence of radical initiators or specific catalysts.
Factors Influencing Product Distribution
Numerous factors beyond the reaction mechanism influence the distribution of major and minor products. These include:
1. Reaction Conditions:
- Temperature: Higher temperatures generally favor reactions with higher activation energies, often leading to different product distributions.
- Solvent: The solvent's polarity significantly impacts the reaction rate and selectivity. Polar solvents favor reactions involving charged intermediates, while nonpolar solvents favor reactions involving neutral species.
- Concentration of Reactants: The relative concentrations of reactants can shift the equilibrium and affect product ratios.
- Presence of Catalysts: Catalysts accelerate reaction rates and can alter the reaction pathway, leading to different products.
- Pressure: Increased pressure can favor reactions that result in a decrease in the number of gas molecules.
2. Steric Hindrance:
Bulky substituents can hinder the approach of reactants, leading to slower reaction rates and influencing product selectivity. This is particularly relevant in SN2 and E2 reactions.
3. Stability of Intermediates and Products:
The stability of carbocations, carbanions, radicals, and other intermediates is crucial in determining product formation. More stable intermediates are formed faster and lead to higher yields of the corresponding products. Thermodynamically more stable products are favored at equilibrium.
4. Kinetic vs. Thermodynamic Control:
Reactions can be under kinetic or thermodynamic control. Kinetic control favors the faster-forming product, regardless of its stability. Thermodynamic control favors the most stable product, even if it forms more slowly. The reaction temperature often dictates which control is dominant.
5. Regioselectivity and Stereoselectivity:
Regioselectivity refers to the preferential formation of one constitutional isomer over another. Stereoselectivity refers to the preferential formation of one stereoisomer over another. Factors like Markovnikov's rule, Zaitsev's rule, and the stereochemistry of the reactants influence these selectivities.
Advanced Techniques for Product Prediction
Predicting the major and minor products often involves analyzing several competing reaction pathways and considering the relative rates of each. Techniques such as Hammond's postulate, which relates the structure of the transition state to the structure of the nearest intermediate, are useful in estimating relative rates.
Computational chemistry provides another powerful tool for predicting reaction outcomes. Using software packages, it's possible to calculate the energies of transition states and intermediates, allowing for accurate prediction of product distributions. However, accurate computational predictions require significant computational resources and expertise.
Examples of Complex Reactions and Product Analysis
Let's consider a few examples illustrating the complexities of predicting major and minor products:
Example 1: Reaction of a chiral alcohol with a strong acid: The reaction of a chiral secondary alcohol with a strong acid can lead to both SN1 and E1 products. The major product will depend on the stability of the carbocation intermediate, the solvent, and the temperature. The stereochemistry of the starting material will also influence the stereochemistry of the products. Rearrangements of the carbocation can also lead to unexpected products.
Example 2: Grignard reaction with an unsymmetrical ketone: The reaction of a Grignard reagent with an unsymmetrical ketone can lead to two different products, depending on which carbonyl carbon the Grignard reagent attacks. Steric hindrance and electronic effects can influence the regioselectivity of the addition.
Example 3: Diels-Alder reaction with substituted dienes and dienophiles: The Diels-Alder reaction is a pericyclic reaction forming a six-membered ring. The regioselectivity and stereoselectivity of the reaction are influenced by the substituents on the diene and dienophile. The endo rule often predicts the major stereoisomer formed.
Conclusion: A Holistic Approach
Predicting the major and minor products of a chemical reaction requires a comprehensive understanding of reaction mechanisms, reaction kinetics, and the influence of various reaction parameters. While no single method guarantees perfect prediction, a combination of mechanistic analysis, consideration of stability, and awareness of the factors controlling regio- and stereoselectivity significantly improves the accuracy of predictions. The use of advanced techniques, such as computational chemistry, can further enhance predictive capabilities. The journey of mastering this skill involves continuous learning and practice, continually refining one's ability to analyze and interpret the complex interplay of factors determining reaction outcomes. This deep understanding is not only essential for synthesizing target molecules efficiently but also for a more comprehensive understanding of chemical processes in general.
Latest Posts
Latest Posts
-
Job A3b Was Ordered By A Customer On September 25
Mar 28, 2025
-
How Can Expectancy Effects Be Reduced
Mar 28, 2025
-
What Ipv4 Address Class Has The Ip Address 221 1 2 3
Mar 28, 2025
-
Economic Growth Rates In Follower Countries
Mar 28, 2025
-
How Might A Wildfire Influence Mass Movement
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Identify The Major And Minor Products Of The Following Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
