Identify The Best Reagents To Achieve The Following Transformation
Holbox
Mar 20, 2025 · 6 min read
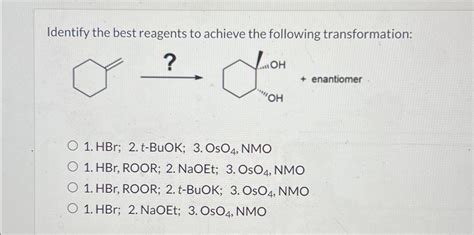
Table of Contents
Identifying the Best Reagents for Organic Transformations: A Comprehensive Guide
Organic synthesis is a fascinating field where the creation of new molecules is driven by the strategic selection of reagents. Choosing the right reagent is paramount to achieving the desired transformation efficiently and selectively. This article delves into the selection process, exploring various factors and providing examples to illustrate how to identify the best reagents for specific transformations. We’ll focus on achieving high yields, minimizing side reactions, and considering practical aspects like cost and safety.
Understanding the Transformation: The Foundation of Reagent Selection
Before diving into potential reagents, a thorough understanding of the desired transformation is crucial. This includes:
1. Defining the Starting Material and Product:
This fundamental step establishes the nature of the transformation. Are we adding functional groups, removing them, or modifying existing ones? Knowing the structure of both the starting material and the target product is the bedrock of reagent selection. For instance, converting an alcohol to an alkyl halide requires different reagents compared to converting an alcohol to a ketone.
2. Identifying the Functional Group Transformation:
Pinpointing the specific functional group transformation is key. Are we performing an oxidation, reduction, alkylation, acylation, or another type of reaction? This immediately narrows down the pool of suitable reagents. For example, oxidizing a primary alcohol to an aldehyde requires milder reagents than oxidizing it to a carboxylic acid.
3. Analyzing the Molecular Structure:
Consider the steric hindrance and electronic effects present in the starting material. Bulky substituents can influence reactivity and regioselectivity. Electron-withdrawing or electron-donating groups can also significantly impact the efficiency and outcome of the reaction. Understanding these factors will help predict which reagents will perform best.
4. Considering Stereochemistry:
If the target molecule has specific stereochemical requirements (e.g., enantioselectivity or diastereoselectivity), then the choice of reagent becomes even more critical. Chiral reagents or catalysts might be necessary to achieve the desired stereochemical outcome. Ignoring stereochemistry can lead to mixtures of isomers, making purification challenging and lowering the overall yield of the desired product.
Common Functional Group Transformations and Reagent Selection:
Let's examine some common functional group transformations and discuss the reagents frequently employed.
1. Oxidation Reactions:
-
Alcohol to Aldehyde/Ketone: The choice here depends on the desired level of oxidation. Pyridinium chlorochromate (PCC) is a mild oxidant that selectively converts primary alcohols to aldehydes and secondary alcohols to ketones. Other options include Dess-Martin periodinane (DMP) and Swern oxidation (using DMSO, oxalyl chloride, and a base). For harsher oxidations leading to carboxylic acids, chromic acid or potassium permanganate can be used.
-
Aldehyde to Carboxylic Acid: This transformation is relatively straightforward. Common reagents include potassium permanganate (KMnO4), chromic acid (H2CrO4), and silver oxide (Ag2O). The choice often depends on the other functional groups present in the molecule, as some reagents can be less selective.
2. Reduction Reactions:
-
Ketone/Aldehyde to Alcohol: Lithium aluminum hydride (LiAlH4) is a powerful reducing agent capable of reducing ketones and aldehydes to alcohols. However, it is highly reactive and requires careful handling. Sodium borohydride (NaBH4) is a milder alternative, suitable for reducing ketones and aldehydes but generally not esters or carboxylic acids.
-
Nitrile to Amine: Lithium aluminum hydride (LiAlH4) is the most commonly used reagent for this reduction. Other options include catalytic hydrogenation using Raney nickel or palladium on carbon.
-
Alkene to Alkane: Catalytic hydrogenation using hydrogen gas (H2) and a metal catalyst like palladium on carbon (Pd/C) or platinum oxide (PtO2) is the standard method for alkene reduction.
3. Alkylation Reactions:
-
Alkylation of Carbonyl Compounds: Grignard reagents (RMgX) and organolithium reagents (RLi) are powerful nucleophiles that react with carbonyl compounds (aldehydes, ketones, esters) to form new carbon-carbon bonds. The choice between Grignard and organolithium reagents often depends on the reactivity and stability of the organometallic reagent. Enolates, generated by treating carbonyl compounds with strong bases like LDA (lithium diisopropylamide), can also be alkylated.
-
SN2 Reactions (Alkyl Halide Substitution): Strong nucleophiles are needed for SN2 reactions. Common examples include hydroxide ion (OH-), alkoxide ions (RO-), azide ion (N3-), cyanide ion (CN-), and thiolate ions (RS-). The choice of nucleophile depends on the desired product and the substrate.
4. Acylation Reactions:
-
Esterification: Carboxylic acids react with alcohols in the presence of an acid catalyst (like sulfuric acid) to form esters. This reaction is an equilibrium process, often requiring the removal of water to drive it towards completion.
-
Acylation of Amines: Acid chlorides or acid anhydrides are commonly used to acylate amines, forming amides. This reaction usually requires a base to neutralize the HCl produced.
Factors Influencing Reagent Selection Beyond Reactivity:
Beyond simply achieving the desired transformation, several other factors must be considered:
1. Selectivity:
A good reagent should react selectively with the target functional group, minimizing side reactions with other functional groups present in the molecule. Regioselectivity (preference for one position over another) and stereoselectivity (preference for one stereoisomer over another) are crucial considerations.
2. Yield:
High yield is essential for efficient synthesis. A reagent that provides a high yield of the desired product is preferable to one that gives a low yield, even if it is more readily available.
3. Cost and Availability:
Reagents vary significantly in cost and availability. A more expensive or less readily available reagent may be justifiable if it offers significant advantages in terms of selectivity, yield, or safety.
4. Safety:
Safety is paramount. Some reagents are highly toxic, flammable, or explosive, requiring special handling procedures. The safety profile of a reagent should be carefully considered before its use.
5. Environmental Impact:
The environmental impact of reagents and solvents should also be a factor in the selection process. Green chemistry principles emphasize the use of environmentally benign reagents and solvents to minimize waste and pollution.
Examples Illustrating Reagent Selection:
Let’s consider a couple of specific examples to illustrate the principles discussed above.
Example 1: Converting a primary alcohol to an aldehyde.
We need a mild oxidant to avoid over-oxidation to the carboxylic acid. PCC (pyridinium chlorochromate) is an excellent choice because it is relatively selective and produces good yields of aldehydes from primary alcohols. Chromic acid, while effective, would likely lead to the carboxylic acid.
Example 2: Preparing an ether via Williamson ether synthesis.
This reaction involves an SN2 reaction between an alkoxide and an alkyl halide. The choice of base to form the alkoxide and the alkyl halide are crucial. A strong base like sodium hydride (NaH) is suitable for forming the alkoxide. The choice of alkyl halide is important because steric hindrance around the carbon bearing the leaving group will affect the rate of the SN2 reaction. Primary alkyl halides react faster than secondary or tertiary alkyl halides.
Conclusion:
Identifying the best reagents for a specific organic transformation requires a multifaceted approach. It involves a thorough understanding of the desired transformation, the properties of the starting materials, the reactivity and selectivity of potential reagents, and practical considerations such as cost, availability, and safety. By carefully considering these factors, chemists can design efficient and selective synthetic routes that lead to high yields of the desired products while minimizing waste and promoting sustainability. This iterative process of understanding, planning, and selection is at the heart of successful organic synthesis. Continued research and development of new reagents are pushing the boundaries of what is possible, creating new opportunities for efficient and environmentally friendly organic chemistry.
Latest Posts
Latest Posts
-
Select All The Differences Between Gymnosperms And Angiosperms
Mar 21, 2025
-
The High Low Formula To Compute Total Costs Is
Mar 21, 2025
-
Carryover Cooking Will Continue To Cook An Item For About
Mar 21, 2025
-
During Its First Year Of Operations
Mar 21, 2025
-
Phrenology Highlighted The Presumed Functions Of
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Identify The Best Reagents To Achieve The Following Transformation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
