Identification Of Selected Anions Lab Answers
Holbox
Mar 15, 2025 · 6 min read
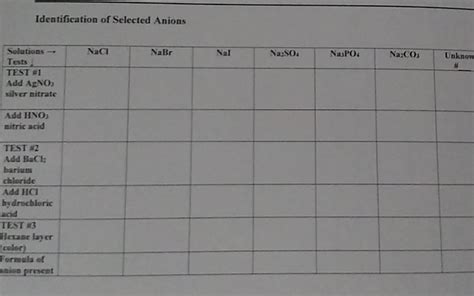
Table of Contents
Identification of Selected Anions: A Comprehensive Lab Guide
Identifying anions in a laboratory setting is a crucial skill in analytical chemistry. This comprehensive guide delves into the common methods used to identify selected anions, providing detailed explanations, practical tips, and troubleshooting advice. We will cover a range of techniques, from simple qualitative tests to more advanced instrumental methods. This guide aims to equip you with the knowledge and confidence to perform accurate anion identification in your laboratory work.
Understanding Anion Identification
Before diving into specific tests, it's vital to understand the underlying principles. Anion identification relies on the unique chemical properties of each anion, allowing them to react differently with specific reagents. These reactions often produce characteristic precipitates, color changes, or gas evolution. The successful identification depends on:
- Careful observation: Note any changes in color, formation of precipitates, evolution of gases, or changes in pH.
- Systematic approach: Follow a logical sequence of tests to avoid confusion and ensure accuracy.
- Control experiments: Always run blank tests (without the sample) to ensure the reagents are pure and to identify any potential interferences.
- Understanding limitations: Each test has its limitations and potential interferences. Be aware of these to interpret the results accurately.
Common Anions and their Identification
This section details the identification of common anions using qualitative tests. Remember to always handle chemicals with care and follow proper laboratory safety procedures.
1. Chloride (Cl⁻)
- Test: Add dilute silver nitrate (AgNO₃) solution to the sample solution. A white precipitate of silver chloride (AgCl) indicates the presence of chloride ions.
- Confirmation: The AgCl precipitate is soluble in dilute ammonia solution but insoluble in nitric acid. This solubility characteristic helps distinguish it from other silver halides.
- Interferences: Bromide and iodide ions will also form precipitates with silver nitrate. These need to be separated and tested for individually.
2. Bromide (Br⁻)
- Test: Add dilute silver nitrate (AgNO₃) solution to the sample solution. A pale yellow precipitate of silver bromide (AgBr) indicates the presence of bromide ions.
- Confirmation: AgBr is insoluble in dilute ammonia solution but slightly soluble in concentrated ammonia solution. This difference in solubility helps distinguish it from chloride and iodide.
- Interferences: Chloride and iodide ions interfere with this test, requiring preliminary separation procedures.
3. Iodide (I⁻)
- Test: Add dilute silver nitrate (AgNO₃) solution to the sample solution. A yellow precipitate of silver iodide (AgI) indicates the presence of iodide ions.
- Confirmation: AgI is insoluble in dilute and concentrated ammonia solutions. This insolubility is a key characteristic for confirming iodide.
- Interferences: Chloride and bromide ions will also form precipitates, necessitating separation steps.
4. Sulfate (SO₄²⁻)
- Test: Add dilute barium chloride (BaCl₂) solution to the sample solution acidified with dilute hydrochloric acid (HCl). A white precipitate of barium sulfate (BaSO₄) indicates the presence of sulfate ions.
- Confirmation: BaSO₄ is insoluble in dilute hydrochloric acid and ammonia solution, helping to distinguish it from other barium salts.
- Interferences: Other anions forming insoluble barium salts may interfere. Careful observation and additional tests are necessary.
5. Carbonate (CO₃²⁻)
- Test: Add dilute hydrochloric acid (HCl) to the sample solution. The evolution of a colorless, odorless gas, carbon dioxide (CO₂), confirms the presence of carbonate ions.
- Confirmation: Bubble the evolved gas through limewater (calcium hydroxide solution). The formation of a white precipitate of calcium carbonate confirms the presence of CO₂.
- Interferences: Sulfites and bicarbonates also react with acids, producing gases. Further tests may be necessary to distinguish between them.
6. Nitrate (NO₃⁻)
- Test: The Brown Ring Test: This test involves adding iron(II) sulfate solution to the sample solution, followed by careful addition of concentrated sulfuric acid down the side of the test tube. A brown ring at the junction of the two layers indicates the presence of nitrate ions.
- Confirmation: The brown ring is due to the formation of a complex ion, [Fe(H₂O)₅NO]²⁺.
- Interferences: Iodide and bromide ions interfere with this test.
7. Phosphate (PO₄³⁻)
- Test: Add ammonium molybdate solution ((NH₄)₆Mo₇O₂₄) to a nitric acid-acidified sample solution. Heating the mixture produces a yellow precipitate of ammonium phosphomolybdate ((NH₄)₃PO₄·12MoO₃) if phosphate ions are present.
- Confirmation: The precipitate's yellow color and formation after heating are characteristic of phosphate.
- Interferences: Arsenate ions can also form a similar precipitate, requiring further tests to distinguish between them.
8. Sulfite (SO₃²⁻)
- Test: Add dilute hydrochloric acid (HCl) to the sample solution. The evolution of sulfur dioxide (SO₂), a pungent, colorless gas, indicates the presence of sulfite ions.
- Confirmation: The SO₂ gas can be identified by its characteristic odor and its ability to decolorize a potassium permanganate solution.
- Interferences: Other anions releasing gases with acids may interfere.
Advanced Techniques for Anion Identification
While qualitative tests are useful for preliminary identification, more advanced techniques provide greater accuracy and sensitivity:
1. Ion Chromatography (IC)
IC is a powerful technique that separates and quantifies anions in a mixture. It employs an ion-exchange column to separate anions based on their affinity for the stationary phase. A detector then measures the concentration of each separated anion. IC offers high sensitivity and accuracy, making it suitable for complex samples.
2. Spectrophotometry
Spectrophotometry can be used to identify and quantify anions based on their absorbance of light at specific wavelengths. This technique is particularly useful for colored anions or those that form colored complexes with specific reagents.
3. Atomic Absorption Spectroscopy (AAS)
While primarily used for cation analysis, AAS can indirectly determine the concentration of some anions by measuring the absorbance of their associated metal cations.
Troubleshooting Common Issues
Several factors can affect the accuracy of anion identification. Here are some common problems and their solutions:
- Interferences: Always consider potential interferences from other ions present in the sample. Separation techniques or masking agents might be necessary.
- Reagent purity: Use high-purity reagents and ensure that your glassware is clean to prevent contamination.
- Incorrect technique: Carefully follow the procedures, paying attention to details like the order of addition of reagents and the required temperature.
- Faulty equipment: Ensure that your equipment (e.g., glassware, heating equipment) is functioning correctly.
- Incorrect interpretation of results: Familiarize yourself with the characteristic reactions and properties of each anion to accurately interpret the results.
Safety Precautions
Always prioritize safety in the laboratory. Remember to:
- Wear appropriate personal protective equipment (PPE), including safety goggles, gloves, and a lab coat.
- Work in a well-ventilated area.
- Handle chemicals carefully and follow proper disposal procedures.
- Be aware of the hazards associated with each chemical used.
- Follow your laboratory's safety regulations and guidelines.
Conclusion
Identifying anions requires a systematic approach, careful observation, and a good understanding of the chemical reactions involved. This guide provides a foundation for identifying common anions using both qualitative and advanced techniques. By mastering these techniques and understanding potential problems, you can accurately identify anions in various samples, contributing to accurate analytical results in your laboratory work. Remember to always consult relevant literature and laboratory manuals for specific details and safety information related to the particular anions and techniques you are employing. Continuous practice and attention to detail are key to becoming proficient in anion identification.
Latest Posts
Latest Posts
-
A Improvement In Production Technology Will Shift The
Mar 15, 2025
-
Select The Nmr Spectrum That Corresponds Best To P Anisidine
Mar 15, 2025
-
An Increase In The Quantity Supplied Suggests A
Mar 15, 2025
-
Libreoffice Is An Example Of Which Type Of Software
Mar 15, 2025
-
The Cash Conversion Cycle Is Computed As
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Identification Of Selected Anions Lab Answers . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
