Hydrogen And Iodine React To Form Hydrogen Iodide Like This
Holbox
Mar 30, 2025 · 5 min read
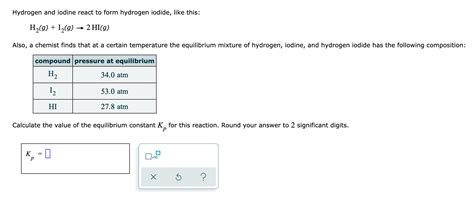
Table of Contents
- Hydrogen And Iodine React To Form Hydrogen Iodide Like This
- Table of Contents
- The Fascinating Reaction: Hydrogen and Iodine Forming Hydrogen Iodide
- Understanding the Reaction: H₂ + I₂ ⇌ 2HI
- The Mechanism: A Detailed Look
- Factors Affecting the Reaction Rate and Equilibrium
- 1. Temperature: The Heat Factor
- 2. Concentration: Reactant Ratios
- 3. Pressure: Influence of Confinement
- 4. Catalyst: Accelerating the Reaction
- 5. Surface Area: Relevance in Heterogeneous Catalysis (Indirect)
- Equilibrium Constant (K<sub>eq</sub>) and its Significance
- Applications and Significance of Hydrogen Iodide
- Latest Posts
- Latest Posts
- Related Post
The Fascinating Reaction: Hydrogen and Iodine Forming Hydrogen Iodide
The reaction between hydrogen gas (H₂) and iodine vapor (I₂) to produce hydrogen iodide (HI) is a classic example of a reversible reaction, demonstrating the principles of chemical equilibrium beautifully. While seemingly simple on the surface, this reaction offers a rich tapestry of insights into kinetics, thermodynamics, and the interplay of factors influencing reaction rates and equilibrium positions. This article delves deep into this reaction, exploring its mechanism, the factors that affect it, and its significance in chemistry.
Understanding the Reaction: H₂ + I₂ ⇌ 2HI
The reaction between hydrogen and iodine is represented by the following reversible equation:
H₂(g) + I₂(g) ⇌ 2HI(g)
This equation indicates that hydrogen gas and iodine vapor react to form hydrogen iodide gas. The double arrow (⇌) signifies that the reaction proceeds in both the forward (formation of HI) and reverse (decomposition of HI) directions simultaneously. At equilibrium, the rates of the forward and reverse reactions are equal, resulting in a constant concentration of reactants and products.
The Mechanism: A Detailed Look
The reaction mechanism for the formation of hydrogen iodide isn't a simple one-step process. While the overall reaction appears straightforward, the actual process involves several elementary steps. The generally accepted mechanism involves a three-step process:
-
Initiation: The reaction begins with the homolytic cleavage of the iodine molecule (I₂) into two iodine atoms (I•). This step requires energy, usually provided by heat. This is a crucial step and is the rate-determining step in the reaction.
-
Propagation: The iodine atom (I•) then reacts with a hydrogen molecule (H₂) to form a hydrogen iodide molecule (HI) and a hydrogen atom (H•). The hydrogen atom (H•) subsequently reacts with another iodine molecule (I₂) to produce another hydrogen iodide molecule (HI) and another iodine atom (I•). This step is crucial for the continuation of the reaction cycle.
-
Termination: The reaction terminates when two iodine atoms (I•) or two hydrogen atoms (H•) collide and combine to form I₂ or H₂, respectively. These reactions remove the free radicals, slowing the reaction down.
This radical mechanism explains why the reaction is relatively slow at lower temperatures. The activation energy required to break the I-I bond is significant, limiting the initial formation of iodine atoms and hence the rate of the reaction.
Factors Affecting the Reaction Rate and Equilibrium
Several factors significantly influence the rate at which the reaction proceeds and the position of the equilibrium:
1. Temperature: The Heat Factor
Increasing the temperature accelerates the reaction. This is because higher temperatures provide more energy to overcome the activation energy barrier required for the homolytic cleavage of the I-I bond, increasing the concentration of reactive iodine atoms (I•) and speeding up the reaction. However, the effect of temperature on the equilibrium constant (K<sub>eq</sub>) is less straightforward and depends on the enthalpy change (ΔH) of the reaction. For this reaction, the forward reaction is slightly exothermic (releases a small amount of heat), meaning that increasing the temperature will shift the equilibrium slightly towards the reactants (favoring the decomposition of HI).
2. Concentration: Reactant Ratios
The concentrations of hydrogen and iodine directly affect the reaction rate. Higher concentrations of reactants lead to more frequent collisions between hydrogen and iodine molecules, increasing the probability of successful reactions and thus the rate of HI formation. The equilibrium position, however, is determined by the ratio of the concentrations of the reactants and products at equilibrium, expressed by the equilibrium constant (K<sub>eq</sub>). Changing the concentration of one reactant will shift the equilibrium to restore the equilibrium constant.
3. Pressure: Influence of Confinement
The reaction involves gaseous reactants and products, making pressure a significant factor. Increasing the pressure will shift the equilibrium towards the side with fewer gas molecules. In this case, since there are two gas molecules on the reactant side and two on the product side, pressure changes have minimal influence on the position of the equilibrium. However, pressure can still affect the reaction rate indirectly by changing the concentration of reactants.
4. Catalyst: Accelerating the Reaction
Catalysts are substances that increase the rate of a reaction without being consumed in the process. While no common catalyst significantly speeds up this specific reaction, it is theoretically possible that a catalyst could lower the activation energy for the bond breaking and radical formation steps, thus accelerating the reaction rate.
5. Surface Area: Relevance in Heterogeneous Catalysis (Indirect)
In a heterogeneous catalytic process, the surface area of the catalyst would influence the reaction rate. However, since this reaction is generally studied in the gas phase (homogeneous), the concept of surface area is less relevant.
Equilibrium Constant (K<sub>eq</sub>) and its Significance
The equilibrium constant (K<sub>eq</sub>) for this reaction is defined as:
K<sub>eq</sub> = [HI]² / ([H₂][I₂])
where [HI], [H₂], and [I₂] represent the equilibrium concentrations of hydrogen iodide, hydrogen, and iodine, respectively. The value of K<sub>eq</sub> indicates the extent to which the reaction proceeds towards the formation of HI at equilibrium. A large K<sub>eq</sub> value suggests that the equilibrium favors the products (HI), while a small K<sub>eq</sub> indicates that the equilibrium favors the reactants (H₂ and I₂). The equilibrium constant's value is temperature-dependent.
Applications and Significance of Hydrogen Iodide
Hydrogen iodide, the product of this reaction, is a highly reactive and versatile compound with numerous applications:
-
Synthesis of Organic Compounds: HI is used as a reducing agent in organic chemistry, facilitating the reduction of various functional groups.
-
Production of Iodides: It serves as a precursor for the synthesis of various metal iodides.
-
Acid Catalyst: HI's acidic nature makes it useful as a catalyst in certain chemical processes.
-
Analytical Chemistry: HI can be used in titrations and other analytical techniques.
The reaction between hydrogen and iodine, seemingly simple, offers a rich learning experience in understanding chemical kinetics and equilibrium principles. The insights gained from studying this reaction are crucial for understanding and predicting the behavior of many other chemical systems. The depth of its exploration allows for the application of numerous concepts in chemical thermodynamics and kinetics, proving its importance in both theoretical and practical chemistry. The factors affecting the reaction rate and equilibrium offer valuable lessons in reaction control and optimization, crucial for industrial chemical processes and laboratory syntheses.
Latest Posts
Latest Posts
-
Refers To A System Under Which The Winning Candidate
Apr 02, 2025
-
A Motor Drives A Pulley And Belt System
Apr 02, 2025
-
Drag The Appropriate Labels To Their Respective Targets Resethelp
Apr 02, 2025
-
Evaluate Each Expression Based On The Following Table
Apr 02, 2025
-
The Primary Objective Of Financial Accounting Is To
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Hydrogen And Iodine React To Form Hydrogen Iodide Like This . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
