How Many Valence Electrons Does A Carbon Atom Have
Holbox
Mar 26, 2025 · 6 min read
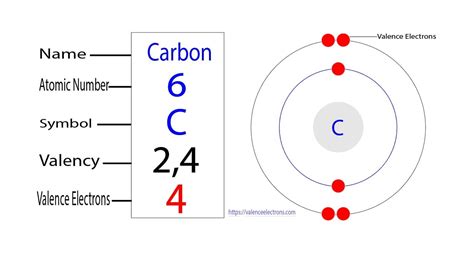
Table of Contents
- How Many Valence Electrons Does A Carbon Atom Have
- Table of Contents
- How Many Valence Electrons Does a Carbon Atom Have? A Deep Dive into Carbon's Bonding Behavior
- Understanding Valence Electrons: The Key to Chemical Bonding
- Carbon's Electronic Configuration: The Foundation of its Tetravalency
- The Importance of Hybridization: Unveiling Carbon's Bonding Versatility
- Other Hybridization Types in Carbon: sp² and sp
- The Vastness of Carbon Chemistry: A Consequence of Four Valence Electrons
- Carbon's Capacity for Chain Formation: The Basis of Organic Macromolecules
- Carbon's Role in Biological Molecules: From Simple Sugars to Complex Proteins
- Conclusion: Carbon's Four Valence Electrons - A Foundation for Life and Beyond
- Latest Posts
- Latest Posts
- Related Post
How Many Valence Electrons Does a Carbon Atom Have? A Deep Dive into Carbon's Bonding Behavior
Carbon. The very word conjures images of diamonds, graphite, and the building blocks of life itself. But what is it about this seemingly simple element that allows it to form such a vast array of compounds? The answer lies in its valence electrons. This article will delve deep into the electronic structure of carbon, explaining why it has four valence electrons, and how this unique characteristic leads to its unparalleled versatility in chemistry and its crucial role in organic chemistry and beyond.
Understanding Valence Electrons: The Key to Chemical Bonding
Before we focus specifically on carbon, let's establish a foundational understanding of valence electrons. Valence electrons are the electrons located in the outermost shell, or energy level, of an atom. These electrons are the primary players in chemical bonding, determining an atom's reactivity and the types of bonds it can form. They are the driving force behind the interactions that create molecules and materials. The number of valence electrons directly influences an atom's chemical behavior and its ability to form stable compounds.
Atoms strive for stability, often achieved by having a full outer shell of electrons. This is frequently described as following the "octet rule," aiming for eight valence electrons (with some exceptions for lighter elements). This pursuit of stability is the fundamental principle governing chemical reactions and the formation of chemical bonds. Atoms will either gain, lose, or share electrons to attain a full outer shell.
Carbon's Electronic Configuration: The Foundation of its Tetravalency
Carbon, with its atomic number of 6, possesses six electrons in total. Its electronic configuration is 1s²2s²2p². This configuration is crucial in understanding why carbon is tetravalent – meaning it has a valency of four. Let's break down this configuration:
-
1s²: Two electrons occupy the first energy level (the innermost shell). These electrons are tightly bound to the nucleus and are generally not involved in chemical bonding. They are considered core electrons.
-
2s²: Two electrons occupy the 2s orbital in the second energy level. These electrons are higher in energy than the 1s electrons and are more accessible for chemical bonding.
-
2p²: Two electrons occupy two of the three 2p orbitals in the second energy level. These orbitals are slightly higher in energy than the 2s orbital. They are also involved in bonding.
Therefore, carbon has four electrons in its outermost shell (the second energy level): two in the 2s orbital and two in the 2p orbitals. These four electrons are its valence electrons. This is why carbon exhibits a valency of four, meaning it can form four covalent bonds with other atoms.
The Importance of Hybridization: Unveiling Carbon's Bonding Versatility
While the simple electronic configuration explains the presence of four valence electrons, it doesn't fully account for carbon's remarkable bonding versatility. To understand this, we need to consider orbital hybridization.
Orbital hybridization is a model that describes the mixing of atomic orbitals within an atom to form new hybrid orbitals. In carbon, the 2s and 2p orbitals hybridize to form four equivalent sp³ hybrid orbitals. These hybrid orbitals are crucial in understanding the geometry and bonding properties of carbon compounds. Each sp³ hybrid orbital contains one electron, making them readily available to form covalent bonds.
This sp³ hybridization leads to the characteristic tetrahedral geometry seen in many carbon compounds, such as methane (CH₄). The four sp³ hybrid orbitals are oriented at an angle of 109.5 degrees to each other, maximizing the distance between electron pairs and minimizing repulsion.
Other Hybridization Types in Carbon: sp² and sp
While sp³ hybridization is common, carbon can also exhibit sp² and sp hybridization, depending on the bonding environment.
-
sp² hybridization: In this case, one 2s orbital and two 2p orbitals hybridize to form three sp² hybrid orbitals, leaving one 2p orbital unhybridized. This hybridization results in a trigonal planar geometry, with bond angles of approximately 120 degrees. This is seen in molecules like ethene (C₂H₄).
-
sp hybridization: Here, one 2s orbital and one 2p orbital hybridize to form two sp hybrid orbitals, leaving two 2p orbitals unhybridized. This hybridization leads to a linear geometry, with bond angles of 180 degrees. This is evident in molecules such as ethyne (C₂H₂).
The Vastness of Carbon Chemistry: A Consequence of Four Valence Electrons
The fact that carbon possesses four valence electrons is fundamental to the immense diversity of organic chemistry. This allows carbon to form strong covalent bonds with a wide range of atoms, including hydrogen, oxygen, nitrogen, sulfur, phosphorus, and even other carbon atoms. This capacity for diverse bonding is responsible for the existence of millions of organic compounds, ranging from simple molecules like methane to complex biomolecules like proteins and DNA.
Carbon's Capacity for Chain Formation: The Basis of Organic Macromolecules
Crucially, carbon atoms can bond to each other to form long chains, branched structures, and rings. This property is unique among elements and is the basis of long-chain hydrocarbons, polymers, and many other important organic molecules. This chain formation allows for the creation of macromolecules, large molecules built from smaller repeating units. These macromolecules are essential to life, playing critical roles in various biological processes.
Carbon's Role in Biological Molecules: From Simple Sugars to Complex Proteins
The four valence electrons of carbon enable it to be the central element in many vital biological molecules.
-
Carbohydrates: These are composed of carbon, hydrogen, and oxygen atoms, often forming long chains or rings. Glucose, a simple sugar crucial for energy production, is a prime example.
-
Lipids: These include fats, oils, and waxes. The carbon backbone of fatty acids forms long hydrocarbon chains, determining their properties.
-
Proteins: These are composed of amino acids, each with a central carbon atom bonded to an amino group, a carboxyl group, a hydrogen atom, and a side chain. The sequence of amino acids dictates the protein's structure and function.
-
Nucleic Acids: DNA and RNA, the carriers of genetic information, are built around a backbone of sugar and phosphate groups, linked together through carbon atoms. The nitrogenous bases that encode genetic information are also based on carbon frameworks.
Conclusion: Carbon's Four Valence Electrons - A Foundation for Life and Beyond
The seemingly simple fact that carbon has four valence electrons has profound implications. This feature underpins carbon's unparalleled ability to form diverse and complex molecules, driving the richness of organic chemistry and the very basis of life itself. From the simplest hydrocarbons to the most intricate biological macromolecules, the chemical behavior of carbon is inextricably linked to its four valence electrons and the versatility of its bonding patterns. Understanding this fundamental characteristic is crucial to grasping the vastness and importance of carbon chemistry across various scientific disciplines. Further research continually reveals the ever-expanding possibilities and implications of carbon's unique bonding capabilities.
Latest Posts
Latest Posts
-
Photosystem I And Photosystem Ii Are Respectively Part Of
Mar 29, 2025
-
Why Is Social Media So Attractive To Consumers
Mar 29, 2025
-
The Industry Low Industry Average And Industry High Cost Benchmarks
Mar 29, 2025
-
The Categories Of Managerial Morality Include
Mar 29, 2025
-
How Is Stress Different From Force
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does A Carbon Atom Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
