Here Are Sketches Of Four Electron Orbitals
Holbox
Mar 16, 2025 · 7 min read
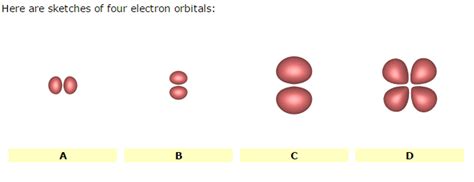
Table of Contents
- Here Are Sketches Of Four Electron Orbitals
- Table of Contents
- Here Are Sketches of Four Electron Orbitals: A Deep Dive into Atomic Structure
- The Quantum Mechanical Model: Understanding Electron Behavior
- Sketching and Understanding the Four Main Orbital Types
- 1. s Orbitals: The Spherical Shells
- 2. p Orbitals: The Dumbbell Shapes
- 3. d Orbitals: More Complex Geometries
- 4. f Orbitals: Even More Intricate Shapes
- Electron Configuration and the Aufbau Principle
- The Significance of Orbital Shapes and Energy Levels
- Beyond the Basics: Hybrid Orbitals and Molecular Orbitals
- Conclusion: A Foundation for Understanding Matter
- Latest Posts
- Latest Posts
- Related Post
Here Are Sketches of Four Electron Orbitals: A Deep Dive into Atomic Structure
Understanding the structure of atoms is fundamental to grasping the principles of chemistry and physics. At the heart of this understanding lies the concept of electron orbitals, regions of space where there's a high probability of finding an electron. This article delves into the fascinating world of electron orbitals, specifically examining four key types: s, p, d, and f orbitals. We'll explore their shapes, energy levels, and how they influence the properties of elements.
The Quantum Mechanical Model: Understanding Electron Behavior
Before diving into the specifics of individual orbitals, it's crucial to understand the framework within which they exist: the quantum mechanical model of the atom. Unlike the simpler Bohr model, which depicts electrons orbiting the nucleus in fixed paths, the quantum mechanical model recognizes the inherent uncertainty in an electron's position and momentum. This uncertainty is encapsulated in Heisenberg's Uncertainty Principle.
Instead of precise orbits, the quantum mechanical model describes electrons using wave functions, which are mathematical descriptions of the electron's probability distribution in space. These probability distributions are represented by electron orbitals. Each orbital is characterized by a set of quantum numbers, which define its size, shape, and orientation in space.
These quantum numbers are:
-
Principal Quantum Number (n): This determines the energy level of the electron and the size of the orbital. It can take on positive integer values (n = 1, 2, 3,...). Higher values of 'n' indicate higher energy levels and larger orbitals.
-
Azimuthal Quantum Number (l): This determines the shape of the orbital and can have integer values from 0 to n-1. Different values of 'l' correspond to different subshells:
- l = 0: s orbital (spherical)
- l = 1: p orbital (dumbbell-shaped)
- l = 2: d orbital (more complex shapes)
- l = 3: f orbital (even more complex shapes)
-
Magnetic Quantum Number (ml): This determines the orientation of the orbital in space. It can have integer values from -l to +l, including 0. For example, a p orbital (l=1) has three possible orientations (ml = -1, 0, +1).
-
Spin Quantum Number (ms): This describes the intrinsic angular momentum of the electron, often visualized as a spin on its axis. It can have only two values: +1/2 or -1/2, representing "spin up" and "spin down".
Sketching and Understanding the Four Main Orbital Types
Now let's examine the four main types of orbitals in more detail:
1. s Orbitals: The Spherical Shells
s orbitals (l=0) are characterized by their spherical shape. The simplest s orbital, the 1s orbital, is a single sphere centered on the nucleus. As the principal quantum number (n) increases, the size of the s orbital increases, but it remains spherical. The higher the 'n' value, the higher the energy level and the greater the distance from the nucleus where the electron is most likely to be found. Think of it as a series of concentric spheres, with each sphere representing a higher energy level. It's important to note that the probability of finding the electron isn't uniformly distributed within the sphere; it's highest at the nucleus and decreases as the distance from the nucleus increases.
Key Characteristics of s Orbitals:
- Shape: Spherical
- Number of orbitals per subshell: 1
- Nodes: Number of radial nodes = n - 1 (where n is the principal quantum number). Radial nodes are regions of zero electron probability within the orbital.
2. p Orbitals: The Dumbbell Shapes
p orbitals (l=1) have a dumbbell shape with two lobes oriented along a specific axis (x, y, or z). For a given principal quantum number (n), there are three p orbitals, each oriented along a different axis. These are often labeled as px, py, and pz. These orbitals are higher in energy than the s orbitals of the same principal quantum number. Imagine three dumbbells, each perpendicular to the others. The probability of finding an electron is highest in the lobes and zero at the nucleus and at the nodal plane between the lobes.
Key Characteristics of p Orbitals:
- Shape: Dumbbell
- Number of orbitals per subshell: 3 (px, py, pz)
- Nodes: Number of radial nodes = n - 2; One nodal plane.
3. d Orbitals: More Complex Geometries
d orbitals (l=2) exhibit more complex shapes than s and p orbitals. There are five d orbitals for each principal quantum number (n ≥ 3). These shapes are less intuitive to visualize, but they possess different orientations in three-dimensional space. They have higher energy than both s and p orbitals of the same principal quantum number.
Four of the five d orbitals have a four-lobed cloverleaf shape, while one has a dumbbell shape with a torus (donut) around the center. Visualizing these shapes requires some spatial reasoning. Understanding their orientations relative to each other is important when discussing chemical bonding and molecular geometry.
Key Characteristics of d Orbitals:
- Shape: Four-lobed cloverleaf or dumbbell with a torus
- Number of orbitals per subshell: 5 (dxy, dyz, dxz, dx²-y², dz²)
- Nodes: Number of radial nodes = n - 3; Two nodal planes or conical nodes.
4. f Orbitals: Even More Intricate Shapes
f orbitals (l=3) possess even more intricate shapes than d orbitals. There are seven f orbitals for each principal quantum number (n ≥ 4). Their complex shapes are even more challenging to visualize and are rarely discussed in introductory chemistry courses. Their complex shapes are attributed to higher angular momentum than the s, p, and d orbitals. These orbitals play a crucial role in the chemistry of lanthanides and actinides, where their influence on chemical bonding and magnetic properties is significant.
Key Characteristics of f Orbitals:
- Shape: Highly complex shapes with multiple lobes and nodes
- Number of orbitals per subshell: 7
- Nodes: Number of radial nodes = n - 4; Three nodal planes or conical nodes
Electron Configuration and the Aufbau Principle
The arrangement of electrons within the orbitals of an atom is described by its electron configuration. The Aufbau principle guides this arrangement: electrons fill orbitals starting with the lowest energy levels and moving upwards. This means that lower energy levels (with lower values of 'n') will be filled before higher energy levels. However, there are exceptions to this rule due to factors like electron-electron repulsion and the relative energies of different subshells.
Hund's rule also plays a significant role in determining electron configurations. It states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This minimizes electron-electron repulsion and leads to more stable configurations.
The Significance of Orbital Shapes and Energy Levels
The shapes and energy levels of orbitals directly influence the chemical behavior of atoms and molecules. The spatial distribution of electrons as determined by the orbital shapes affects how atoms interact with each other, forming chemical bonds. The energy levels of the orbitals determine the amount of energy required to remove an electron from an atom (ionization energy) and the energy released or absorbed when electrons transition between energy levels. This has profound implications in spectroscopy, where the absorption and emission of light can reveal crucial information about the electronic structure of atoms and molecules.
Beyond the Basics: Hybrid Orbitals and Molecular Orbitals
This discussion has primarily focused on atomic orbitals. However, in molecules, atomic orbitals combine to form molecular orbitals, which describe the behavior of electrons in the entire molecule. Hybrid orbitals are also crucial in understanding molecular geometry. These concepts represent a more advanced level of understanding and are best explored in subsequent studies of molecular structure and bonding.
Conclusion: A Foundation for Understanding Matter
The study of electron orbitals is fundamental to our understanding of atomic structure and the properties of matter. While visualizing the more complex orbitals, such as d and f orbitals, can be challenging, grasping the basic concepts of their shapes, energy levels, and how they influence chemical behavior is crucial for developing a strong foundation in chemistry and related fields. This article provided a comprehensive overview of the four principal orbital types—s, p, d, and f—and their significance in determining the properties of atoms and molecules. Continued exploration of these concepts, along with the study of molecular orbitals and hybridization, will provide a deeper understanding of the complex world of atomic and molecular structure.
Latest Posts
Latest Posts
-
Where Should Glassware Be Stored After It Is Cleaned
Mar 17, 2025
-
A Favorable Labor Rate Variance Indicates That
Mar 17, 2025
-
Split The Worksheet Into Panes At Cell D16
Mar 17, 2025
-
A Winning Strategy Is One That
Mar 17, 2025
-
The Objective Of Inventory Management Is To
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Here Are Sketches Of Four Electron Orbitals . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
