H C C H Lewis Structure
Holbox
Apr 03, 2025 · 6 min read
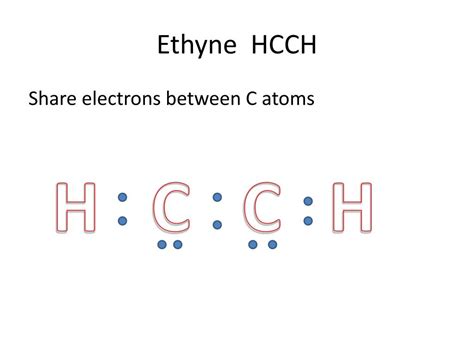
Table of Contents
- H C C H Lewis Structure
- Table of Contents
- Unveiling the Secrets of the H₂CCH₂ Lewis Structure: A Comprehensive Guide
- Constructing the H₂CCH₂ Lewis Structure: A Step-by-Step Approach
- Beyond the Basic Structure: Delving Deeper into Ethene's Bonding
- Understanding Sigma (σ) and Pi (π) Bonds
- Hybridization in Ethene: sp² Hybridization
- What is sp² Hybridization?
- The Geometry of Ethene: Planar Structure
- Ethene's Reactivity: Implications of the Double Bond
- Common Reactions of Ethene:
- Spectroscopic Analysis of Ethene: Confirming the Structure
- Techniques that provide insights:
- The Significance of Ethene: A Versatile Building Block
- Industrial Applications:
- Conclusion: A Deeper Appreciation for a Simple Molecule
- Latest Posts
- Latest Posts
- Related Post
Unveiling the Secrets of the H₂CCH₂ Lewis Structure: A Comprehensive Guide
The humble molecule, ethene (also known as ethylene, H₂CCH₂), might appear simple at first glance. However, understanding its Lewis structure reveals a fascinating world of bonding, geometry, and reactivity. This comprehensive guide delves deep into the intricacies of the H₂CCH₂ Lewis structure, exploring its construction, implications, and significance in chemistry. We'll unravel the mysteries behind its double bond, delve into its hybridization, and examine how its structure dictates its properties and chemical behavior.
Constructing the H₂CCH₂ Lewis Structure: A Step-by-Step Approach
Before we begin, it's crucial to understand the fundamental principles underlying Lewis structure drawing. Lewis structures, also known as Lewis dot diagrams, visually represent the valence electrons of atoms in a molecule and how they are shared to form covalent bonds.
Step 1: Counting Valence Electrons
The first and most important step is determining the total number of valence electrons in the molecule. Carbon (C) has four valence electrons, and Hydrogen (H) has one. In ethene (H₂CCH₂), we have two carbons and four hydrogens. Therefore, the total number of valence electrons is:
(2 x 4 electrons/carbon) + (4 x 1 electron/hydrogen) = 12 valence electrons
Step 2: Identifying the Central Atom(s)
In ethene, carbon atoms are less electronegative than hydrogen atoms, making them the central atoms. These two carbon atoms will form a bond with each other.
Step 3: Arranging Atoms and Forming Single Bonds
We arrange the two carbon atoms next to each other and connect them with a single bond (represented by a line). This uses two of our twelve valence electrons. Then, we connect each carbon atom to two hydrogen atoms with single bonds, using another eight electrons.
H H
| |
C - C
| |
H H
Step 4: Completing the Octet Rule
After forming single bonds, we have four valence electrons remaining. The octet rule (with the exception of hydrogen, which follows the duet rule) states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight valence electrons. Currently, each carbon atom only has six electrons (two from the C-C bond and four from the C-H bonds). To satisfy the octet rule for both carbon atoms, we need to add a double bond between the two carbons. This involves sharing two more electron pairs, using our remaining four valence electrons.
H H
| |
C = C
| |
H H
Step 5: Verifying the Structure
Our final Lewis structure shows each hydrogen atom with two electrons (satisfying the duet rule) and each carbon atom with eight electrons (satisfying the octet rule). All twelve valence electrons have been used. This structure accurately depicts the bonding in ethene.
Beyond the Basic Structure: Delving Deeper into Ethene's Bonding
The H₂CCH₂ Lewis structure reveals a crucial feature: the carbon-carbon double bond. This double bond consists of one sigma (σ) bond and one pi (π) bond.
Understanding Sigma (σ) and Pi (π) Bonds
-
Sigma (σ) bond: This is a strong, single bond formed by the direct head-on overlap of atomic orbitals. In ethene, the C-C sigma bond and all the C-H sigma bonds are formed this way.
-
Pi (π) bond: This is a weaker bond formed by the sideways overlap of p orbitals. In ethene, the pi bond exists above and below the plane of the sigma bonds, strengthening the overall C=C bond.
The presence of a double bond significantly impacts the properties of ethene, making it more reactive than alkanes (which only have single bonds).
Hybridization in Ethene: sp² Hybridization
To fully understand the geometry and bonding in ethene, we need to consider the concept of hybridization. The carbon atoms in ethene are sp² hybridized.
What is sp² Hybridization?
In sp² hybridization, one s orbital and two p orbitals of a carbon atom combine to form three sp² hybrid orbitals. These hybrid orbitals are arranged in a trigonal planar geometry with bond angles of approximately 120°. The remaining p orbital remains unhybridized and participates in the formation of the pi (π) bond.
The Geometry of Ethene: Planar Structure
Due to the sp² hybridization and the presence of a double bond, ethene exhibits a planar structure. All six atoms (four hydrogens and two carbons) lie in the same plane. This planarity is crucial for the effective sideways overlap of p orbitals to form the pi (π) bond. Any deviation from planarity would weaken the pi bond and destabilize the molecule.
Ethene's Reactivity: Implications of the Double Bond
The presence of the carbon-carbon double bond makes ethene significantly more reactive than alkanes. The pi bond is relatively weaker and more exposed than the sigma bonds, making it susceptible to various reactions.
Common Reactions of Ethene:
-
Addition Reactions: In addition reactions, the pi bond breaks, and new atoms or groups are added across the double bond. Examples include:
- Halogenation: Reaction with halogens (like chlorine or bromine) to form dihaloalkanes.
- Hydrogenation: Reaction with hydrogen gas in the presence of a catalyst (like platinum or palladium) to form ethane.
- Hydration: Reaction with water in the presence of an acid catalyst to form ethanol.
-
Polymerization: Ethene molecules can undergo addition polymerization to form polyethylene, a widely used plastic. This involves the repeated addition of ethene monomers to form long chains.
Spectroscopic Analysis of Ethene: Confirming the Structure
Various spectroscopic techniques can confirm the structure of ethene and its double bond.
Techniques that provide insights:
-
Infrared (IR) Spectroscopy: The presence of a C=C double bond shows characteristic absorption bands in the IR spectrum.
-
Nuclear Magnetic Resonance (NMR) Spectroscopy: ¹H NMR and ¹³C NMR can provide information about the chemical environment of the hydrogen and carbon atoms in ethene.
-
Ultraviolet (UV) Spectroscopy: The pi (π) electrons in the double bond absorb UV light at specific wavelengths, providing further evidence for the double bond's presence.
The Significance of Ethene: A Versatile Building Block
Ethene is not just a molecule of academic interest; it’s a crucial building block in the chemical industry. Its versatility stems from its reactivity and ability to undergo a wide range of transformations.
Industrial Applications:
-
Polyethylene Production: Ethene is the primary feedstock for the production of polyethylene, a widely used plastic in various applications.
-
Ethylene Oxide Production: Ethene is used to produce ethylene oxide, a key intermediate in the production of various chemicals, including antifreeze and detergents.
-
Other Derivatives: Ethene serves as a precursor for numerous other important chemicals and materials.
Conclusion: A Deeper Appreciation for a Simple Molecule
The seemingly simple H₂CCH₂ Lewis structure actually holds immense information about the bonding, geometry, reactivity, and industrial significance of ethene. By carefully constructing the Lewis structure and understanding the underlying principles of bonding, hybridization, and spectroscopic techniques, we gain a deeper appreciation for this fundamental molecule and its importance in chemistry and industry. This detailed exploration hopefully provides a comprehensive understanding of ethene's molecular structure and its far-reaching implications. From its simple visual representation to its complex chemical behavior, ethene exemplifies the power of understanding molecular structure to predict and manipulate chemical properties.
Latest Posts
Latest Posts
-
A Workstation Is Out Of Compliance
Apr 06, 2025
-
Gold Forms A Substitutional Solid Solution With Silver
Apr 06, 2025
-
Over Longer Periods Of Time Demand Tends To Become
Apr 06, 2025
-
The Scientific Study Of Life Called
Apr 06, 2025
-
The Carbon Nitrogen Peptide Bond Is Rigid
Apr 06, 2025
Related Post
Thank you for visiting our website which covers about H C C H Lewis Structure . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
