Given The Planar Trisubstituted Cyclohexane Fill In The Missing Substituents
Holbox
Mar 19, 2025 · 5 min read
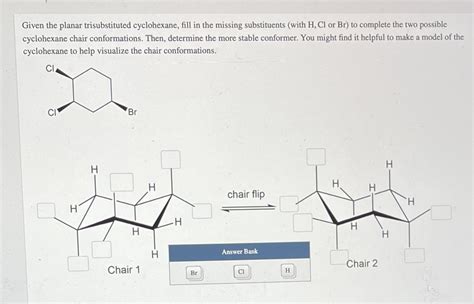
Table of Contents
Given a Planar Trisubstituted Cyclohexane: Filling in the Missing Substituents
Determining the configuration of substituents on a cyclohexane ring is a fundamental concept in organic chemistry. While cyclohexane itself adopts a chair conformation to minimize steric strain, planar representations are frequently used for simplicity, especially when dealing with trisubstituted cyclohexanes. However, a planar representation inherently lacks the crucial information about axial and equatorial positions, essential for fully defining the stereochemistry. This article will explore the methods and reasoning behind deducing the missing substituents on a planar trisubstituted cyclohexane, highlighting the importance of understanding conformational analysis and stereochemical principles.
Understanding Cyclohexane Conformations
Before tackling trisubstituted cyclohexanes, it's crucial to grasp the fundamental conformations of cyclohexane: the chair and boat conformations. The chair conformation is significantly more stable due to the staggered arrangement of its carbon-hydrogen bonds, minimizing torsional strain and steric interactions. The boat conformation is significantly less stable due to flagpole interactions and torsional strain.
Within the chair conformation, substituents can occupy either axial or equatorial positions. Axial substituents are oriented parallel to the vertical axis of the ring, while equatorial substituents lie approximately in the plane of the ring. The relative stability of chair conformers with different substituent arrangements plays a crucial role in determining the preferred conformation and ultimately, the observed stereochemistry.
Analyzing Planar Representations of Trisubstituted Cyclohexanes
A planar representation of a trisubstituted cyclohexane, while simplifying the visualization, obscures the crucial information about the axial and equatorial positions of the substituents. To determine the missing substituents, we must consider several factors:
1. Cis-Trans Relationships:
The relative orientations of substituents (cis or trans) are crucial. Cis substituents are on the same side of the ring, while trans substituents are on opposite sides. This information, often explicitly stated or implied in the problem, guides the assignment of axial and equatorial positions.
2. Applying the Principles of Stability:
The most stable conformation of a substituted cyclohexane is the one that places the largest number of bulky substituents in equatorial positions. This is governed by the principle of minimizing 1,3-diaxial interactions, which are unfavorable steric interactions between axial substituents and hydrogen atoms on the same side of the ring.
3. Considering the Size of Substituents:
The size and bulkiness of the substituents significantly influence the stability of the conformations. Larger groups prefer equatorial positions more strongly than smaller groups. This preferential occupation is crucial when dealing with multiple substituents. For example, a tert-butyl group will almost exclusively occupy an equatorial position.
4. Drawing Chair Conformations:
To deduce the missing substituents, converting the planar representation into a chair conformation is essential. This process involves systematically placing the known substituents in either axial or equatorial positions, guided by the cis/trans relationships and principles of stability. Multiple chair conformations might be drawn, but only the most stable conformation will accurately reflect the stereochemistry.
5. Evaluating 1,3-Diaxial Interactions:
Once chair conformations are drawn, examine the interactions between axial substituents and hydrogen atoms on the same side of the ring. Conformations with fewer 1,3-diaxial interactions are more stable.
Worked Examples:
Let's consider a few examples to solidify these concepts. Assume we are given the planar projection of a trisubstituted cyclohexane:
Example 1: A planar representation shows a cyclohexane ring with a methyl group (CH3) at position 1 and a chlorine atom (Cl) at position 3. The relationship between these two substituents is cis. This means they must be on the same side of the cyclohexane ring in the chair conformation.
- Step 1: Start by drawing a cyclohexane chair conformation.
- Step 2: Place the methyl group at position 1 (either axial or equatorial).
- Step 3: Since the methyl and chlorine are cis, place the chlorine atom on the same side of the ring as the methyl group, in the same orientation (either axial or equatorial).
- Step 4: Evaluate both possible conformations (one with both substituents axial, the other with both equatorial). The conformation with both substituents equatorial will be more stable.
Example 2: A planar projection shows a cyclohexane with a bromine (Br) at position 1, an ethyl group (CH2CH3) at position 3, and the relationship between them is trans.
- Step 1: Draw the cyclohexane chair.
- Step 2: Place the bromine atom at position 1 (either axial or equatorial).
- Step 3: Since the bromine and ethyl group are trans, place the ethyl group on the opposite side of the ring from the bromine. This requires one to be axial and the other equatorial.
- Step 4: The more stable conformation will have the bulkier ethyl group in the equatorial position.
Example 3: Incorporating a third substituent Let's add a hydroxyl group (-OH) at position 4, with its relative configuration to the Bromine and ethyl group specified. The task then requires careful placement of the -OH group, ensuring the specified cis/trans relationships are maintained and favoring the most stable conformation (minimizing 1,3-diaxial interactions).
Example 4: Dealing with ambiguity: Sometimes, the planar projection might not provide sufficient information to definitively determine the complete stereochemistry. For instance, if only two substituents and their cis/trans relationship are specified, several possible configurations could exist. In such cases, additional information is required, such as NMR data or detailed reaction schemes that illustrate stereoselectivity.
Advanced Considerations:
-
Conformation Interconversion: Cyclohexane chair conformations interconvert through a process involving ring flipping. This process changes the axial and equatorial positions of substituents. The relative energies of the conformers determine the equilibrium population of each. Understanding this interconversion is crucial for analyzing more complex systems.
-
Anomeric Effect: In molecules containing oxygen or nitrogen atoms, the anomeric effect can influence the preferred conformation. This effect is caused by the stabilization of a gauche conformation of certain groups, and it overrides the standard steric preferences.
Conclusion:
Determining the missing substituents on a planar trisubstituted cyclohexane requires a thorough understanding of conformational analysis and stereochemical principles. By systematically applying the principles of cis/trans relationships, stability considerations, and minimizing 1,3-diaxial interactions, one can accurately deduce the complete stereochemical information and draw the most stable chair conformation. Remembering that larger groups prefer equatorial positions is a valuable rule of thumb, but remembering exceptions caused by effects like the anomeric effect is also important for achieving mastery of the topic. While planar representations provide a simplified view, the transition to chair conformations is crucial for a complete and accurate depiction of the molecular structure and stereochemistry. Further practice with varied examples and progressively complex scenarios will hone your skills in successfully tackling these problems.
Latest Posts
Latest Posts
-
The Understatement Of The Ending Inventory Balance Causes
Mar 19, 2025
-
Why Do Women Not Figure More Prominently Among Early Sociologists
Mar 19, 2025
-
Which Of The Following Is An Example Of Positive Feedback
Mar 19, 2025
-
Is Composed Of A Network Of Branching Elastic Fibers
Mar 19, 2025
-
How Are These Terms Related Plausible Believable
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Given The Planar Trisubstituted Cyclohexane Fill In The Missing Substituents . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
