For Each Solute Identify The Better Solvent
Holbox
Mar 16, 2025 · 6 min read
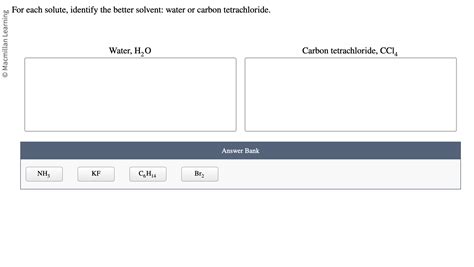
Table of Contents
For Each Solute, Identify the Better Solvent: A Comprehensive Guide to Solvent Selection
Choosing the right solvent is crucial in numerous chemical processes, from simple dissolution to complex reactions. The effectiveness of a solvent depends heavily on the nature of the solute. "Like dissolves like" is a fundamental principle: polar solutes tend to dissolve in polar solvents, and nonpolar solutes dissolve in nonpolar solvents. However, understanding the nuances of intermolecular forces and the specific properties of both solute and solvent is key to making the best selection. This article delves into the principles of solubility and provides a detailed guide to selecting the optimal solvent for various solutes.
Understanding the "Like Dissolves Like" Principle
The adage "like dissolves like" is a concise summary of solubility principles. It highlights the importance of the intermolecular forces present in both the solute and the solvent. Solutes dissolve effectively when the attractive forces between solute and solvent molecules are strong enough to overcome the attractive forces within the solute itself and the attractive forces within the solvent.
-
Polar Solvents: These solvents possess a significant dipole moment, meaning they have a separation of positive and negative charge within the molecule. Examples include water (H₂O), ethanol (C₂H₅OH), and acetone (CH₃COCH₃). Polar solvents effectively dissolve polar solutes, such as ionic compounds (salts) and polar organic molecules (alcohols, carboxylic acids, amines). The strong dipole-dipole interactions and hydrogen bonding (in the case of water and alcohols) are crucial for solvation.
-
Nonpolar Solvents: These solvents have a symmetrical distribution of charge, resulting in a negligible or zero dipole moment. Examples include hexane (C₆H₁₄), benzene (C₆H₆), and carbon tetrachloride (CCl₄). Nonpolar solvents dissolve nonpolar solutes, such as oils, fats, and many organic compounds with predominantly carbon-hydrogen bonds. The primary intermolecular forces involved are weak London dispersion forces.
Key Intermolecular Forces and Their Influence on Solubility
Several types of intermolecular forces dictate solubility. Understanding these forces is paramount in solvent selection:
-
Ion-Dipole Forces: These strong forces exist between ions (charged particles) and polar molecules. They play a critical role in dissolving ionic compounds in polar solvents like water. The positive ends of water molecules attract anions, while the negative ends attract cations, effectively separating the ions and allowing them to dissolve.
-
Dipole-Dipole Forces: These forces arise between polar molecules due to their permanent dipoles. The positive end of one molecule attracts the negative end of another, leading to mutual attraction and solvation. The strength of dipole-dipole forces increases with increasing polarity.
-
Hydrogen Bonding: A special type of dipole-dipole interaction involving hydrogen atoms bonded to highly electronegative atoms like oxygen, nitrogen, or fluorine. Hydrogen bonding is particularly strong and is responsible for the high boiling point and excellent solvent properties of water.
-
London Dispersion Forces (LDFs): These weak forces are present in all molecules, but they are the primary intermolecular force in nonpolar molecules. They arise from temporary fluctuations in electron distribution, creating temporary dipoles that induce dipoles in neighboring molecules. The strength of LDFs increases with molecular size and surface area.
Practical Examples of Solute-Solvent Matching
Let's examine several examples to illustrate the selection of appropriate solvents:
1. Sodium Chloride (NaCl) - An Ionic Solute
Solute: Sodium chloride is an ionic compound with strong electrostatic interactions between Na⁺ and Cl⁻ ions.
Better Solvent: Water (H₂O). The strong ion-dipole forces between the ions and water molecules effectively overcome the ionic lattice energy, allowing NaCl to dissolve readily. Organic solvents, even polar ones, are generally poor solvents for NaCl due to the weaker interactions.
2. Benzene (C₆H₆) - A Nonpolar Solute
Solute: Benzene is a nonpolar aromatic hydrocarbon with only London dispersion forces between its molecules.
Better Solvent: Hexane (C₆H₁₄) or other nonpolar solvents. Hexane, also a nonpolar molecule, interacts with benzene primarily through London dispersion forces. These forces are sufficient to overcome the intermolecular forces in benzene, allowing it to dissolve. Polar solvents will not effectively dissolve benzene due to the lack of significant interactions.
3. Ethanol (C₂H₅OH) - A Polar Solute
Solute: Ethanol is a polar molecule with both dipole-dipole interactions and hydrogen bonding capabilities.
Better Solvent: Water (H₂O). Water, with its strong hydrogen bonding capability, interacts favorably with ethanol's hydroxyl (-OH) group. This leads to strong solute-solvent interactions, facilitating dissolution. While ethanol can dissolve in some other polar solvents, water is typically the best choice due to its strong hydrogen bonding capacity.
4. Iodine (I₂) - A Nonpolar Solute
Solute: Iodine is a nonpolar molecule with relatively weak London dispersion forces.
Better Solvent: Carbon tetrachloride (CCl₄). Both iodine and carbon tetrachloride are nonpolar, and the interactions between them (London dispersion forces) are sufficient to dissolve iodine. Polar solvents would not be effective due to the lack of substantial interactions.
5. Sucrose (C₁₂H₂₂O₁₁) - A Polar Solute
Solute: Sucrose (table sugar) is a polar molecule containing numerous hydroxyl (-OH) groups capable of hydrogen bonding.
Better Solvent: Water (H₂O). The numerous hydroxyl groups in sucrose allow for extensive hydrogen bonding with water molecules, resulting in high solubility. Other polar solvents might dissolve some sucrose, but water's superior hydrogen bonding capacity makes it the best solvent.
Factors Beyond "Like Dissolves Like"
While the "like dissolves like" principle is a useful guideline, other factors influence solubility:
-
Temperature: Solubility often increases with increasing temperature, as the kinetic energy of molecules overcomes intermolecular forces. However, this is not always the case; some substances exhibit decreased solubility with increased temperature.
-
Pressure: Pressure significantly affects the solubility of gases in liquids. Increasing pressure increases the solubility of gases according to Henry's Law. The effect on the solubility of solids and liquids is usually negligible.
-
Solvent Mixtures: The solubility of a solute can be enhanced by using a mixture of solvents, even if one of the solvents alone is not a good solvent for the solute. This technique is common in organic chemistry to achieve desired solubility.
Advanced Considerations for Solvent Selection
For more complex applications, selecting a solvent requires additional considerations:
-
Toxicity: The toxicity of the solvent must be assessed, especially for applications involving human contact or environmental release. Choosing less toxic solvents is crucial for safety and environmental responsibility.
-
Flammability: The flammability of the solvent should be considered, particularly in industrial settings where fire hazards are a concern. Choosing non-flammable solvents can significantly reduce the risk of fire.
-
Cost: Solvent cost is an important economic factor, especially for large-scale applications. Choosing cost-effective solvents without compromising performance is essential.
-
Ease of Recovery: The ease of solvent recovery after the process is complete should be considered. Solvents that can be easily evaporated or distilled are preferred for economic and environmental reasons.
Conclusion: A Holistic Approach to Solvent Selection
Choosing the right solvent involves a careful assessment of both the solute and the desired properties of the solution. While the "like dissolves like" principle provides a solid foundation, understanding the subtleties of intermolecular forces, temperature effects, and practical considerations is crucial for making the best selection. Prioritizing safety, cost-effectiveness, and environmental impact ensures responsible and efficient solvent usage across various chemical processes. By carefully weighing these factors, researchers and professionals can optimize their processes and achieve desired results. Remember that this is a guide, and thorough research and experimentation may be necessary to determine the optimal solvent for a specific application.
Latest Posts
Latest Posts
-
A Customer Arrives At A Customer Service Desk
Mar 17, 2025
-
Which Of The Following Best Describes How Deviance Is Defined
Mar 17, 2025
-
What Products Are Expected In The Ethoxide Promoted
Mar 17, 2025
-
Cups And Glasses Are Taking Too Long
Mar 17, 2025
-
The Most Significant Hazard Associated With Splinting Is
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about For Each Solute Identify The Better Solvent . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
