Experiment 5 Kinetics: The Oxidation Of Iodide By Hydrogen Peroxide
Holbox
Mar 25, 2025 · 6 min read
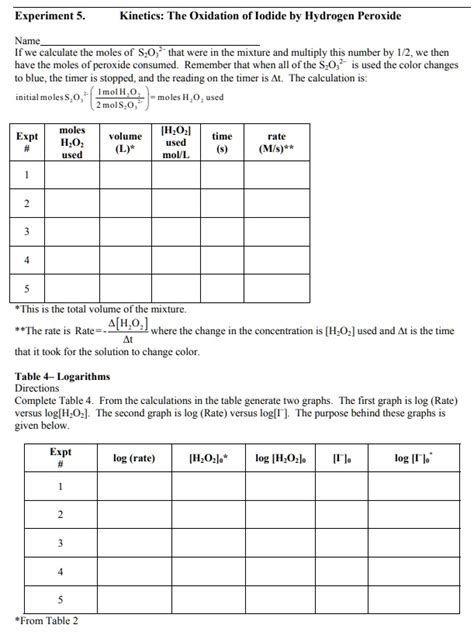
Table of Contents
- Experiment 5 Kinetics: The Oxidation Of Iodide By Hydrogen Peroxide
- Table of Contents
- Experiment 5 Kinetics: The Oxidation of Iodide by Hydrogen Peroxide
- Understanding the Reaction
- Experimental Procedure
- 1. Preparation of Solutions:
- 2. Experimental Runs:
- 3. Data Analysis:
- Determining the Rate Law
- Potential Sources of Error
- Safety Precautions
- Advanced Analysis and Extensions
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Experiment 5 Kinetics: The Oxidation of Iodide by Hydrogen Peroxide
This comprehensive guide delves into the intricacies of Experiment 5: Kinetics – The Oxidation of Iodide by Hydrogen Peroxide. We will explore the theoretical background, practical procedures, data analysis, and potential sources of error, providing a robust understanding of this fundamental chemical kinetics experiment.
Understanding the Reaction
The reaction between iodide ions (I⁻) and hydrogen peroxide (H₂O₂) in acidic conditions is a classic example used to study chemical kinetics. It's a redox reaction where iodide is oxidized to iodine (I₂), and hydrogen peroxide is reduced. The overall reaction can be represented as:
H₂O₂(aq) + 2I⁻(aq) + 2H⁺(aq) → I₂(aq) + 2H₂O(l)
This reaction's rate is dependent on the concentrations of the reactants: hydrogen peroxide, iodide ions, and hydrogen ions (from the acid). The experiment aims to determine the rate law, which expresses the relationship between the reaction rate and the concentrations of these reactants. The general rate law for this reaction takes the form:
Rate = k[H₂O₂]ˣ[I⁻]ʸ[H⁺]ᶻ
Where:
- k is the rate constant
- x, y, and z are the reaction orders with respect to H₂O₂, I⁻, and H⁺, respectively.
The experiment focuses on determining the values of x, y, and z through a series of carefully designed experiments. By systematically varying the concentrations of each reactant while keeping others constant, we can observe the effect on the reaction rate and thus determine the individual reaction orders.
Experimental Procedure
The experiment typically involves using a spectrophotometer to monitor the reaction's progress. The appearance of iodine (I₂) is monitored by measuring its absorbance at a specific wavelength (usually around 460 nm). The absorbance is directly proportional to the concentration of I₂, which allows us to track the reaction rate.
Here's a typical procedure outline:
1. Preparation of Solutions:
- Prepare a stock solution of hydrogen peroxide (H₂O₂) of known concentration.
- Prepare a stock solution of potassium iodide (KI), providing a source of iodide ions.
- Prepare a stock solution of a strong acid, such as sulfuric acid (H₂SO₄), to provide the acidic environment.
- Prepare a stock solution of sodium thiosulfate (Na₂S₂O₃) as a crucial component of the reaction mixture. This is because the reaction is monitored by the rate of appearance of I2. Thiosulfate ions react rapidly with the iodine produced, maintaining a constant concentration of free I2.
2. Experimental Runs:
Several experimental runs are conducted with varying concentrations of H₂O₂, KI, and H⁺, while keeping the others constant in each run. This systematic variation allows us to determine the reaction orders. A typical experiment would involve the following steps for each run:
- Mixing the Reactants: Carefully measure the required volumes of H₂O₂, KI, H₂SO₄, and Na₂S₂O₃ solutions and mix them in a cuvette. The starch solution is added to indicate the endpoint.
- Spectrophotometer Measurement: Immediately place the cuvette into the spectrophotometer and begin recording the absorbance at the chosen wavelength (460 nm) over time. The data will consist of absorbance readings as a function of time.
- Data Recording: Record the absorbance readings at regular intervals (e.g., every 30 seconds) for several minutes. This gives you a detailed record of the reaction progress.
3. Data Analysis:
The data collected (absorbance versus time) will be used to determine the rate of the reaction. This will then be used to determine the orders of reaction with respect to each reactant. The common method is the initial rates method.
This involves plotting the initial rate of the reaction (which is proportional to the initial slope of the absorbance-time curve) versus the concentration of each reactant, while keeping the concentrations of other reactants constant.
Determining the Rate Law
The data obtained from multiple experimental runs, where the concentrations of reactants are systematically varied, allows for the determination of the rate law's exponents (x, y, and z).
Initial Rates Method: This is a common method used to analyze kinetic data. By comparing the initial rates of reaction from different runs, we can deduce the order of each reactant. For instance:
- If doubling the concentration of a reactant doubles the initial rate, the reaction is first-order with respect to that reactant (x = 1, for example).
- If doubling the concentration of a reactant quadruples the initial rate, the reaction is second-order with respect to that reactant (x = 2, for example).
- If changing the concentration of a reactant has no effect on the initial rate, the reaction is zero-order with respect to that reactant (x = 0, for example).
Potential Sources of Error
Several factors can introduce errors into the experiment:
- Temperature Fluctuations: Temperature significantly affects reaction rates. Maintaining a constant temperature throughout the experiment is crucial.
- Impurities in Reagents: Impurities in the reactants can catalyze or inhibit the reaction, leading to inaccurate rate measurements.
- Mixing Errors: Incomplete mixing of the reactants can result in non-uniform concentrations, affecting the reaction rate.
- Spectrophotometer Calibration: An improperly calibrated spectrophotometer can lead to inaccurate absorbance readings.
- Reaction Time: The time taken to mix the reactants and insert the cuvette into the spectrophotometer can cause variation in initial measurement, and errors in initial rate.
- Incomplete Reaction: If the reaction does not go to completion, the measured absorbance may not accurately represent the final concentration of iodine.
Safety Precautions
Always follow standard laboratory safety procedures when performing this experiment. These include:
- Eye Protection: Wear safety goggles at all times.
- Appropriate Clothing: Wear a lab coat and gloves.
- Handling of Chemicals: Handle all chemicals with care, following the instructions on their respective safety data sheets. Hydrogen peroxide is an oxidizer, and sulfuric acid is corrosive.
- Waste Disposal: Dispose of chemical waste according to your laboratory's guidelines.
Advanced Analysis and Extensions
Beyond determining the rate law, this experiment offers opportunities for more in-depth analysis. For example:
- Determining the activation energy (Ea): By conducting the experiment at different temperatures, the activation energy can be determined using the Arrhenius equation.
- Investigating the effect of catalysts: The impact of various catalysts on the reaction rate can be studied.
- Exploring the reaction mechanism: The rate law can provide insights into the reaction mechanism, suggesting the sequence of elementary steps involved in the overall reaction.
Conclusion
The oxidation of iodide by hydrogen peroxide is a versatile experiment that provides valuable hands-on experience in chemical kinetics. By carefully following the experimental procedure, analyzing the data correctly, and understanding potential sources of error, students can gain a comprehensive understanding of reaction rates, rate laws, and the factors influencing them. This experiment is not only a crucial exercise in understanding chemical kinetics but also serves as a foundation for more advanced studies in physical chemistry and reaction mechanisms. The detailed understanding of experimental design, data analysis, and error handling provides a solid foundation for future scientific endeavors. Through careful attention to detail and a systematic approach, this experiment can offer a rich learning experience, furthering understanding of chemical kinetics and experimental methodology. Remember to always prioritize safety and follow all appropriate laboratory procedures.
Latest Posts
Latest Posts
-
Provide A Systematic Name For The Following Compound
Mar 26, 2025
-
What Is The Minimum Hot Holding Temperature For Baked Potatoes
Mar 26, 2025
-
Label The Parts Of The Hair And Hair Follicle
Mar 26, 2025
-
A Public Opinion Poll In Ohio Wants To Determine
Mar 26, 2025
-
Which Item Does Not Have Food Contact Surface
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Experiment 5 Kinetics: The Oxidation Of Iodide By Hydrogen Peroxide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
