Provide A Systematic Name For The Following Compound
Holbox
Mar 26, 2025 · 7 min read
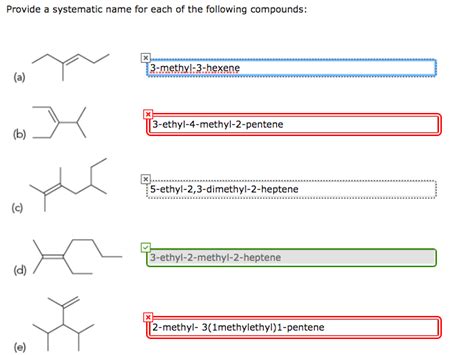
Table of Contents
- Provide A Systematic Name For The Following Compound
- Table of Contents
- Providing Systematic Names for Chemical Compounds: A Comprehensive Guide
- Understanding IUPAC Nomenclature
- Naming Inorganic Compounds
- Binary Ionic Compounds (Two Elements)
- Ternary Ionic Compounds (Three or More Elements)
- Acids
- Naming Organic Compounds
- Alkanes: The Foundation of Organic Nomenclature
- Branched Alkanes
- Alkenes and Alkynes
- Functional Groups
- Complex Organic Compounds
- Practical Application and Resources
- Latest Posts
- Latest Posts
- Related Post
Providing Systematic Names for Chemical Compounds: A Comprehensive Guide
Naming chemical compounds, also known as chemical nomenclature, is a crucial aspect of chemistry. A systematic name uniquely identifies a compound, allowing chemists worldwide to understand and communicate about specific molecules without ambiguity. This article delves into the principles of systematic naming, focusing on various compound types and providing a step-by-step approach to derive their IUPAC (International Union of Pure and Applied Chemistry) names. We'll explore the nuances of naming organic and inorganic compounds, ensuring clarity and precision in the process.
Understanding IUPAC Nomenclature
The International Union of Pure and Applied Chemistry (IUPAC) is the globally recognized authority for standardizing chemical nomenclature. The IUPAC system provides a logical and unambiguous way to name compounds based on their structure. This systematic approach eliminates confusion caused by common or trivial names, which may vary regionally or even refer to multiple compounds. Adhering to IUPAC rules is vital for clear communication and accurate representation of chemical structures in research, industry, and education.
Naming Inorganic Compounds
Inorganic compounds encompass a vast range of substances excluding those primarily composed of carbon-hydrogen bonds (organic compounds). Their naming conventions often differ from organic compounds and rely on identifying the constituent elements and their oxidation states.
Binary Ionic Compounds (Two Elements)
Binary ionic compounds consist of a metal cation and a non-metal anion. The naming process is straightforward:
-
Name the cation (metal) first. The name remains the same as the element's name. For transition metals exhibiting multiple oxidation states (e.g., iron, copper), the oxidation state is indicated using Roman numerals in parentheses after the metal's name. For example, Fe²⁺ is iron(II), and Fe³⁺ is iron(III).
-
Name the anion (non-metal) second. The root name of the non-metal is used, with the suffix "-ide" added. For example, chlorine becomes chloride, oxygen becomes oxide, and sulfur becomes sulfide.
Examples:
- NaCl: Sodium chloride
- MgO: Magnesium oxide
- FeCl₂: Iron(II) chloride
- FeCl₃: Iron(III) chloride
- Cu₂O: Copper(I) oxide
- CuO: Copper(II) oxide
Ternary Ionic Compounds (Three or More Elements)
Ternary ionic compounds typically involve a metal cation and a polyatomic anion (an ion composed of two or more atoms). The naming process is similar to binary compounds:
-
Name the cation (metal) first. Follow the same rules as above for transition metals with multiple oxidation states.
-
Name the polyatomic anion second. Use the standard name for the polyatomic ion (e.g., sulfate (SO₄²⁻), nitrate (NO₃⁻), phosphate (PO₄³⁻), hydroxide (OH⁻), ammonium (NH₄⁺)).
Examples:
- Na₂SO₄: Sodium sulfate
- Ca(NO₃)₂: Calcium nitrate
- (NH₄)₃PO₄: Ammonium phosphate
- Fe(OH)₃: Iron(III) hydroxide
Acids
Acids are compounds that release hydrogen ions (H⁺) when dissolved in water. Their names depend on the anion they form when the hydrogen ion is removed:
-
Binary acids: These contain hydrogen and a non-metal. The name starts with "hydro-", followed by the root name of the non-metal with the suffix "-ic acid". For example, HCl is hydrochloric acid, and H₂S is hydrosulfuric acid.
-
Oxoacids: These contain hydrogen, a non-metal, and oxygen. The naming depends on the oxidation state of the central non-metal atom:
- If the non-metal has a higher oxidation state, the suffix "-ic acid" is used. For example, HNO₃ is nitric acid, and H₂SO₄ is sulfuric acid.
- If the non-metal has a lower oxidation state, the suffix "-ous acid" is used. For example, HNO₂ is nitrous acid, and H₂SO₃ is sulfurous acid.
Naming Organic Compounds
Organic compounds are based on carbon and hydrogen, often with other elements like oxygen, nitrogen, sulfur, and halogens. Their naming is more complex than inorganic compounds and follows a set of rules based on the compound's structure and functional groups.
Alkanes: The Foundation of Organic Nomenclature
Alkanes are saturated hydrocarbons (only single bonds between carbon atoms). They form the basis for naming many other organic compounds. The first four alkanes have trivial names: methane (CH₄), ethane (C₂H₆), propane (C₃H₈), and butane (C₄H₁₀). For alkanes with five or more carbon atoms, the names are derived from Greek prefixes indicating the number of carbons, followed by "-ane": pentane (C₅H₁₂), hexane (C₆H₁₄), heptane (C₇H₁₆), and so on.
Branched Alkanes
Branched alkanes have carbon atoms branching off the main carbon chain. Their names are determined by:
-
Identifying the longest continuous carbon chain: This chain forms the parent alkane name.
-
Numbering the carbon atoms in the longest chain: Numbering starts from the end closest to the first substituent (branch).
-
Naming the substituents (branches): Alkyl groups are named by replacing the "-ane" ending of the alkane with "-yl" (e.g., methyl, ethyl, propyl, butyl).
-
Locating the substituents: The position of each substituent is indicated by the number of the carbon atom it is attached to.
-
Combining the information: The name includes the substituent names (alphabetically ordered), their locations, and the parent alkane name. Numbers are separated by commas, and numbers and words are separated by hyphens.
Example:
Consider the compound with the structure: CH₃-CH(CH₃)-CH₂-CH₃
- Longest chain: 4 carbons (butane)
- Numbering: Start from the left to give the substituent the lowest number.
- Substituent: Methyl (CH₃) at carbon 2.
- Name: 2-methylbutane
Alkenes and Alkynes
Alkenes contain at least one carbon-carbon double bond, while alkynes contain at least one carbon-carbon triple bond. Their naming is similar to alkanes, but with the following modifications:
- Suffix: "-ene" for alkenes, "-yne" for alkynes.
- Locant: The position of the double or triple bond is indicated by a number before the suffix. The numbering starts from the end closest to the multiple bond.
Example:
CH₂=CH-CH₂-CH₃ is 1-butene.
Functional Groups
Functional groups are specific groups of atoms within molecules that determine their chemical properties and reactivity. Many organic compounds are named based on the presence of these functional groups. Some common functional groups include:
-
Alcohols (-OH): The suffix "-ol" is added to the parent alkane name. The position of the hydroxyl group (-OH) is indicated by a number. For example, CH₃CH₂OH is ethanol.
-
Aldehydes (-CHO): The suffix "-al" is added. For example, CH₃CHO is ethanal.
-
Ketones (C=O): The suffix "-one" is added. The position of the carbonyl group (C=O) is indicated by a number. For example, CH₃COCH₃ is propanone (acetone).
-
Carboxylic Acids (-COOH): The suffix "-oic acid" is added. For example, CH₃COOH is ethanoic acid (acetic acid).
-
Amines (-NH₂): The suffix "-amine" is added. For example, CH₃NH₂ is methanamine.
-
Ethers (R-O-R'): The names of the alkyl groups are listed alphabetically, followed by "ether". For example, CH₃OCH₃ is dimethyl ether.
Complex Organic Compounds
Naming complex organic molecules involving multiple functional groups or ring systems can become significantly more challenging, often requiring a detailed understanding of IUPAC nomenclature rules and prioritization of functional groups. Specific rules for naming various classes of compounds (e.g., cyclic compounds, aromatic compounds, heterocyclic compounds) are needed. Consult advanced organic chemistry textbooks or IUPAC guidelines for detailed information on these complex structures.
Practical Application and Resources
To effectively name chemical compounds, practice is crucial. Start with simple examples, gradually increasing the complexity. Numerous online resources and textbooks offer practice problems and detailed explanations of IUPAC nomenclature. Furthermore, chemical drawing software often incorporates IUPAC naming functionalities, assisting in the generation of systematic names from drawn structures. Remember, consistency and attention to detail are paramount when applying IUPAC nomenclature to avoid ambiguity and ensure accurate communication within the scientific community.
This comprehensive guide provides a solid foundation for understanding and applying IUPAC nomenclature. By mastering these principles, you can confidently assign and interpret systematic names for a wide range of chemical compounds, facilitating clear and unambiguous communication in the field of chemistry. Continuous practice and consulting reliable resources will further enhance your skills in this essential area of chemistry.
Latest Posts
Latest Posts
-
The Demand Curve For A Monopoly Is
Mar 30, 2025
-
This Month Carla Caught The Following Fish
Mar 30, 2025
-
Most Of Our Energy Waste In North America Results From
Mar 30, 2025
-
A Bondholder That Owns A 1000 10 10 Year Bond Has
Mar 30, 2025
-
A Nurse Is Caring For A Client
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Provide A Systematic Name For The Following Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
