Draw The Structure Of 3-ethyl-5 5-dimethylcyclohexene
Holbox
Mar 29, 2025 · 6 min read
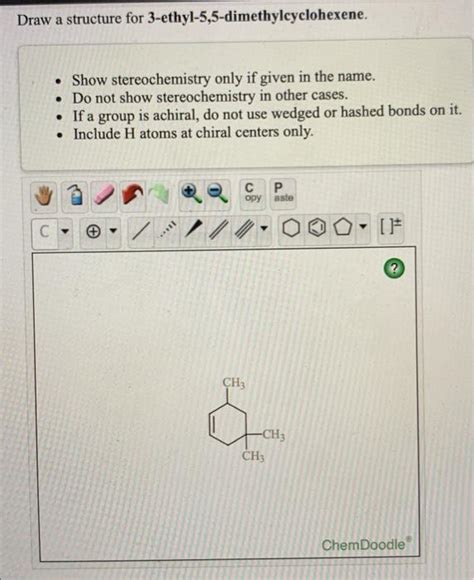
Table of Contents
- Draw The Structure Of 3-ethyl-5 5-dimethylcyclohexene
- Table of Contents
- Drawing the Structure of 3-Ethyl-5,5-Dimethylcyclohexene: A Comprehensive Guide
- Understanding the IUPAC Name
- Step-by-Step Drawing Process
- Isomerism Explained: Exploring the Possibilities
- Advanced Considerations: Stereochemistry and Conformations
- Applications and Importance
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Drawing the Structure of 3-Ethyl-5,5-Dimethylcyclohexene: A Comprehensive Guide
Drawing organic molecules can seem daunting, especially when dealing with complex structures like 3-ethyl-5,5-dimethylcyclohexene. However, with a systematic approach and understanding of nomenclature, the process becomes significantly easier. This comprehensive guide will walk you through drawing this specific molecule, explaining each step in detail and providing insights into the principles of organic chemistry nomenclature.
Understanding the IUPAC Name
Before we start drawing, let's break down the IUPAC name: 3-ethyl-5,5-dimethylcyclohexene. This name tells us everything we need to know to construct the molecule:
-
cyclohexene: This indicates a six-membered carbon ring containing a double bond (alkene). The
-enesuffix signifies the presence of a carbon-carbon double bond. -
5,5-dimethyl: This signifies two methyl groups (–CH₃) attached to the fifth carbon atom of the cyclohexene ring. The "5,5" indicates that both methyl groups are on the same carbon.
-
3-ethyl: This indicates an ethyl group (–CH₂CH₃) attached to the third carbon atom of the cyclohexene ring.
Step-by-Step Drawing Process
Now, let's construct the molecule step-by-step:
Step 1: Draw the Cyclohexene Ring
Begin by drawing a hexagon representing the cyclohexene ring. Remember that cyclohexene is a cyclic structure, meaning the carbon atoms form a closed ring.
1
/ \
2---3
| |
6---4
5
Step 2: Locate the Double Bond
Cyclohexene, by definition, contains one double bond. While the IUPAC name doesn't specify its location, the numbering convention dictates that we give the double bond the lowest possible numbers. Therefore, we place the double bond between carbons 1 and 2.
1=2
/ \
3---4
| |
6---5
Step 3: Add the Substituents
Now, let's add the substituents:
-
5,5-dimethyl: Add two methyl groups (–CH₃) to carbon 5.
-
3-ethyl: Add an ethyl group (–CH₂CH₃) to carbon 3.
1=2
/ \
3-CH2CH3---4
| |
6---5-(CH3)2
Step 4: Complete the Structure
Finally, add the remaining hydrogen atoms to satisfy the valency of each carbon atom. Remember that carbon atoms always form four bonds. Each carbon atom in the ring (except for those in the double bond) will have two hydrogen atoms attached. The carbons in the double bond each have one hydrogen atom. The methyl groups have three hydrogen atoms each, and the ethyl group has five hydrogen atoms.
H H
| |
H-C1=C2-H
| |
H-C3-CH2CH3 H
| |
H-C6-C5(CH3)2 H
|
H
Step 5: Consider Isomers (Optional but Important)
While the name 3-ethyl-5,5-dimethylcyclohexene implies a specific structure, it's crucial to understand the possibility of isomers. Isomers are molecules with the same molecular formula but different arrangements of atoms. In this case, the position of the ethyl and methyl groups could, theoretically, affect the molecule's three-dimensional structure, though in this specific case the differences are minor. Understanding the concept of isomerism is key for advanced organic chemistry.
Isomerism Explained: Exploring the Possibilities
The molecule 3-ethyl-5,5-dimethylcyclohexene is relatively simple, but it highlights some important isomeric concepts:
1. Constitutional Isomerism: Constitutional isomers are isomers that have different connectivity of atoms. While the name 3-ethyl-5,5-dimethylcyclohexene specifies a particular connectivity, it's possible to imagine other constitutional isomers, such as molecules with the ethyl group on a different carbon atom, or with the methyl groups located at different positions. For example, a 1-ethyl-3,3-dimethylcyclohexene would be a constitutional isomer.
2. Stereoisomerism: Stereoisomers possess the same connectivity of atoms but differ in the spatial arrangement of atoms. Two main types of stereoisomerism are relevant here:
-
Geometric Isomerism (cis-trans): Due to the double bond, geometric isomerism is possible. The cis isomer has both the ethyl and the methyl groups on the same side of the double bond, while the trans isomer has them on opposite sides. This is an important consideration for the molecule's properties and reactions.
-
Conformational Isomerism: Cyclohexane rings are not planar. They exist in different conformations (chair, boat, twist-boat), which are interconverting isomers that differ only in their bond rotations. The methyl and ethyl groups could adopt different orientations within these conformations affecting their energy and stability.
Advanced Considerations: Stereochemistry and Conformations
Understanding the three-dimensional structure of 3-ethyl-5,5-dimethylcyclohexene requires delving into stereochemistry and conformational analysis.
Stereochemistry: This branch of chemistry deals with the spatial arrangement of atoms in a molecule and how this affects its properties. The molecule's stereochemistry is influenced by the double bond's geometry and the positions of the substituents.
Conformational Analysis: Cyclohexane rings prefer chair conformations due to their lower energy. In the chair conformation, the substituents can occupy either axial (up or down) or equatorial positions. The equatorial position is generally more stable for larger substituents like ethyl and methyl groups due to less steric hindrance. Therefore, the most stable conformation of 3-ethyl-5,5-dimethylcyclohexene would likely have the ethyl and methyl groups in predominantly equatorial positions. Analyzing the possible chair conformations helps determine the molecule's overall stability and reactivity.
Applications and Importance
Understanding the structure of molecules like 3-ethyl-5,5-dimethylcyclohexene is crucial in many fields:
-
Organic Synthesis: Chemists need to accurately represent molecules to plan and execute chemical reactions. The structure informs synthetic strategies, allowing researchers to design pathways to synthesize complex compounds.
-
Drug Discovery: Many drugs are organic molecules with specific structures crucial for their biological activity. The ability to accurately draw and understand molecular structures enables scientists to design and optimize new drugs.
-
Materials Science: Many materials' properties depend on their molecular structure. The ability to understand and depict structures is vital in designing new materials with desired characteristics.
-
Spectroscopy: Techniques like NMR and IR spectroscopy rely heavily on understanding molecular structure. The predicted spectra of 3-ethyl-5,5-dimethylcyclohexene can be compared to experimental results to confirm the structure.
Conclusion
Drawing the structure of 3-ethyl-5,5-dimethylcyclohexene may seem complex initially, but a systematic approach, rooted in the understanding of IUPAC nomenclature, is key to success. By breaking down the name, step-by-step construction, considering the possibilities of isomerism, and understanding the principles of stereochemistry and conformational analysis, you can confidently represent the molecule's structure, along with its different possibilities, accurately. This ability is fundamental to a deeper understanding of organic chemistry and its broad applications. This article aimed to equip you with the knowledge and skills to not only draw this specific molecule but also apply the principles to other organic structures, facilitating your journey through the fascinating world of organic chemistry.
Latest Posts
Latest Posts
-
Is Balloon Vine Bigger Than Golden Rain Tree
Apr 01, 2025
-
Unused Live Ammunition Should Be Inventoried And Then
Apr 01, 2025
-
The Discount On Bonds Payable Account
Apr 01, 2025
-
Moles And Chemical Formulas Report Sheet Answers
Apr 01, 2025
-
The Narrower The Definition Of A Product
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Draw The Structure Of 3-ethyl-5 5-dimethylcyclohexene . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
