Draw The Organic Product Of The Nucleophilic Substitution Reaction
Holbox
Mar 18, 2025 · 6 min read
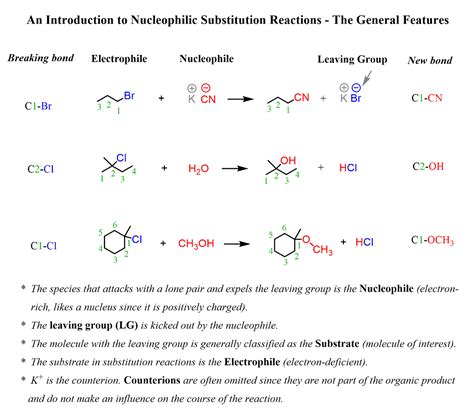
Table of Contents
Drawing the Organic Product of Nucleophilic Substitution Reactions: A Comprehensive Guide
Nucleophilic substitution reactions are fundamental in organic chemistry, forming the backbone of countless synthetic pathways. Understanding these reactions and accurately predicting the organic product is crucial for any aspiring chemist. This comprehensive guide will delve into the intricacies of nucleophilic substitution, providing a step-by-step approach to drawing the organic product and highlighting key considerations.
Understanding Nucleophilic Substitution Reactions
Nucleophilic substitution, as the name suggests, involves the substitution of one nucleophile for another in an organic molecule. A nucleophile is an electron-rich species that is attracted to positively charged or partially positively charged atoms (electrophilic centers). The reaction typically involves a leaving group, an atom or group of atoms that departs from the molecule taking a pair of electrons with it. There are two main mechanisms for nucleophilic substitution: SN1 and SN2.
SN2 Reactions (Bimolecular Nucleophilic Substitution)
SN2 reactions are concerted reactions, meaning the bond breaking and bond forming occur simultaneously in a single step. The nucleophile attacks the substrate from the backside, opposite the leaving group. This backside attack leads to inversion of configuration at the chiral center (if present). The rate of an SN2 reaction depends on the concentration of both the substrate and the nucleophile – hence, it's bimolecular.
Factors Favoring SN2 Reactions:
- Strong nucleophiles: Nucleophiles with a negative charge or lone pairs of electrons readily available for donation.
- Primary substrates: Primary alkyl halides (R-X, where R is a primary alkyl group and X is a leaving group) are favored as steric hindrance is minimal.
- Aprotic solvents: Solvents that don't have an acidic proton (e.g., DMSO, DMF, acetone). These solvents don't solvate the nucleophile, keeping it reactive.
SN1 Reactions (Unimolecular Nucleophilic Substitution)
SN1 reactions proceed through a two-step mechanism. The first step involves the departure of the leaving group, forming a carbocation intermediate. This is the rate-determining step, and its rate depends only on the concentration of the substrate – hence, unimolecular. The second step involves the nucleophile attacking the carbocation. Since the carbocation is planar, the nucleophile can attack from either side, leading to a racemic mixture of products if the starting material was chiral.
Factors Favoring SN1 Reactions:
- Weak nucleophiles: Nucleophiles that are less reactive and won't compete with the leaving group departure.
- Tertiary substrates: Tertiary alkyl halides are favored due to the stability of the tertiary carbocation. The alkyl groups stabilize the positive charge.
- Protic solvents: Solvents with acidic protons (e.g., water, alcohols). These solvents stabilize the carbocation intermediate.
Drawing the Organic Product: A Step-by-Step Guide
Regardless of the mechanism (SN1 or SN2), drawing the organic product involves identifying the nucleophile, leaving group, and the electrophilic carbon center undergoing substitution. Let's break down the process:
Step 1: Identify the Nucleophile and Leaving Group
This is the crucial first step. Common leaving groups include halides (Cl⁻, Br⁻, I⁻), tosylates (OTs), mesylates (OMs), and water. Stronger bases are generally better leaving groups. Identify the nucleophile based on its electron-rich nature; it often possesses a lone pair or a negative charge.
Step 2: Identify the Electrophilic Carbon
The carbon atom bonded to the leaving group is the electrophilic carbon. This is where the nucleophile will attack.
Step 3: Determine the Reaction Mechanism (SN1 or SN2)
Consider the factors mentioned earlier – the nature of the nucleophile, the substrate structure (primary, secondary, tertiary), and the solvent. This will determine whether the reaction proceeds via SN1 or SN2.
Step 4: Draw the Product based on the Mechanism
-
SN2: The nucleophile attacks from the backside, resulting in inversion of configuration at the chiral center (if present). The leaving group departs simultaneously.
-
SN1: The leaving group departs first to form a carbocation. The nucleophile then attacks the carbocation from either side, potentially leading to a racemic mixture if the carbocation is chiral.
Step 5: Consider Stereochemistry
Stereochemistry is crucial in nucleophilic substitution. Remember that SN2 reactions cause inversion of configuration, while SN1 reactions can lead to racemization. Clearly indicate the stereochemistry in your drawn product using wedged and dashed bonds.
Examples: Drawing the Products
Let's illustrate this with some examples.
Example 1: SN2 Reaction
Consider the reaction of bromomethane (CH₃Br) with sodium hydroxide (NaOH) in ethanol.
- Nucleophile: OH⁻
- Leaving Group: Br⁻
- Substrate: CH₃Br (primary alkyl halide, favoring SN2)
- Mechanism: SN2
The product will be methanol (CH₃OH), with inversion of configuration (though not relevant in this achiral case).
Example 2: SN1 Reaction
Consider the reaction of tert-butyl bromide ((CH₃)₃CBr) with water.
- Nucleophile: H₂O
- Leaving Group: Br⁻
- Substrate: (CH₃)₃CBr (tertiary alkyl halide, favoring SN1)
- Mechanism: SN1
The first step involves the departure of Br⁻, forming a tert-butyl carbocation. Water then attacks the carbocation, followed by deprotonation to give tert-butyl alcohol ((CH₃)₃COH). Since the carbocation is not chiral, racemization is not a concern here.
Example 3: SN2 Reaction with a Chiral Center
Consider the reaction of (S)-2-bromobutane with sodium cyanide (NaCN) in acetone.
- Nucleophile: CN⁻
- Leaving Group: Br⁻
- Substrate: (S)-2-bromobutane (secondary alkyl halide, but the strong nucleophile and aprotic solvent favour SN2)
- Mechanism: SN2
The product will be (R)-2-cyanobutane due to the inversion of configuration at the chiral center. Remember to draw the product with the correct stereochemistry.
Example 4: SN1 Reaction with a Chiral Center
Consider the reaction of (R)-2-bromo-2-methylbutane with methanol (CH₃OH).
- Nucleophile: CH₃OH
- Leaving Group: Br⁻
- Substrate: (R)-2-bromo-2-methylbutane (tertiary alkyl halide, favoring SN1)
- Mechanism: SN1
The product will be a racemic mixture of (R)- and (S)-2-methoxy-2-methylbutane. The carbocation intermediate is planar and allows attack from either side with equal probability.
Advanced Considerations
- Ambident Nucleophiles: Some nucleophiles have multiple nucleophilic sites (e.g., CN⁻ can attack through either C or N). The regioselectivity depends on the reaction conditions.
- Competition between SN1 and SN2: In some cases, both SN1 and SN2 mechanisms can compete. The predominant mechanism depends on the relative rates of each pathway.
- Rearrangements: Carbocation rearrangements can occur in SN1 reactions if a more stable carbocation can be formed. This leads to unexpected products.
- Leaving Group Ability: A good leaving group should be able to stabilize the negative charge after departing.
Conclusion
Mastering the ability to predict and draw the organic product of nucleophilic substitution reactions is paramount for success in organic chemistry. By carefully considering the nucleophile, leaving group, substrate structure, and solvent, you can accurately determine the mechanism (SN1 or SN2) and draw the correct product, including its stereochemistry. Remember to practice diligently using a variety of examples, and you will become proficient in this essential aspect of organic chemistry. Continuously reviewing the key factors and mechanistic details will enhance your understanding and problem-solving skills. This will lay a solid foundation for tackling more complex organic reactions and synthetic strategies.
Latest Posts
Latest Posts
-
Under What Circumstances Should A Companys Management Team
Mar 18, 2025
-
Who Can Apply Pesticides In A Food Service Establishment
Mar 18, 2025
-
A Nation Can Increase Its Production Possibilities By
Mar 18, 2025
-
Which Of The Following Mixtures Are Solutions
Mar 18, 2025
-
The Dupont Identity Can Be Accurately Defined As
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Draw The Organic Product Of The Nucleophilic Substitution Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
