Draw The Major Organic Product Of The Reaction Shown Below
Holbox
Mar 20, 2025 · 6 min read
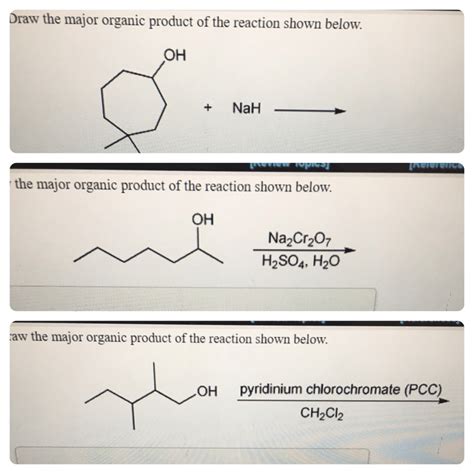
Table of Contents
Draw the Major Organic Product of the Reaction Shown Below: A Comprehensive Guide
Predicting the major organic product of a given reaction is a fundamental skill in organic chemistry. This requires a deep understanding of reaction mechanisms, functional group transformations, and the interplay of various factors influencing reaction pathways. This article delves into the process, providing a structured approach to tackling such problems, and illustrating with diverse examples. We will explore common reaction types, including nucleophilic substitutions, electrophilic additions, eliminations, and rearrangements, equipping you with the tools to confidently predict the major product in a variety of scenarios.
Understanding Reaction Mechanisms: The Key to Predicting Products
Before diving into specific examples, it's crucial to understand the underlying mechanisms driving organic reactions. Mechanisms detail the step-by-step process of bond breaking and bond formation, providing a molecular-level explanation for the transformation. Knowing the mechanism helps predict not only the product but also the stereochemistry (3D arrangement of atoms) of the product.
Common Reaction Mechanisms:
-
SN1 (Substitution Nucleophilic Unimolecular): This mechanism proceeds through a carbocation intermediate. It's favored by tertiary substrates, protic solvents, and weak nucleophiles. The reaction is stereochemically non-specific, leading to racemization.
-
SN2 (Substitution Nucleophilic Bimolecular): This is a concerted mechanism, meaning bond breaking and bond formation occur simultaneously. It's favored by primary substrates, aprotic solvents, and strong nucleophiles. The reaction proceeds with inversion of configuration.
-
E1 (Elimination Unimolecular): Similar to SN1, this mechanism involves a carbocation intermediate. It's favored by tertiary substrates, high temperatures, and protic solvents.
-
E2 (Elimination Bimolecular): A concerted mechanism involving the simultaneous removal of a leaving group and a proton. It's favored by strong bases and often leads to specific stereochemical outcomes (e.g., Zaitsev's rule).
-
Electrophilic Addition: This mechanism is common for alkenes and alkynes, involving the addition of an electrophile across the multiple bond. Markovnikov's rule often governs the regioselectivity (which carbon atom the electrophile adds to).
-
Electrophilic Aromatic Substitution: Aromatic rings undergo substitution reactions, where an electrophile replaces a hydrogen atom. The directing effects of substituents on the ring play a crucial role in determining the position of substitution.
Analyzing Reaction Conditions: The Context Matters
The reaction conditions—solvent, temperature, concentration of reactants, and the presence of catalysts—significantly influence the outcome of a reaction.
Solvent Effects:
- Protic solvents (e.g., water, alcohols) can stabilize carbocations and participate in hydrogen bonding.
- Aprotic solvents (e.g., DMF, DMSO) are less likely to interfere with the reaction mechanism.
Temperature:
Higher temperatures often favor elimination reactions over substitution reactions.
Reagent Concentration:
High concentrations of strong nucleophiles favor SN2 and E2 reactions.
Predicting the Major Product: A Step-by-Step Approach
Let's break down the process of predicting the major organic product of a given reaction:
-
Identify the Functional Groups: Recognize the functional groups present in the starting material and reagents. This is the foundation for determining the likely reaction type.
-
Determine the Reaction Type: Based on the functional groups and reagents, predict the most likely reaction type (SN1, SN2, E1, E2, addition, etc.).
-
Consider the Mechanism: Understand the step-by-step mechanism of the predicted reaction. This is critical for determining the structure of the intermediate(s) and the final product.
-
Analyze Reaction Conditions: Assess the reaction conditions (solvent, temperature, concentration) to determine their influence on the reaction pathway and product formation.
-
Predict the Major Product: Based on the mechanism and reaction conditions, predict the structure of the major product, including its stereochemistry.
-
Consider Regioselectivity and Stereoselectivity: Determine if the reaction shows any preference for the formation of one regioisomer or stereoisomer over others.
Examples and Detailed Explanations
Let's illustrate the above approach with several examples:
Example 1: SN1 Reaction
(CH3)3CBr + H2O → ?
-
Functional Groups: Tertiary alkyl halide and water.
-
Reaction Type: SN1 (favored by tertiary alkyl halides and a protic solvent like water).
-
Mechanism: The reaction proceeds through a carbocation intermediate formed by the departure of the bromide ion. Water then acts as a nucleophile, attacking the carbocation. Proton transfer yields the alcohol.
-
Reaction Conditions: Protic solvent (water) favors SN1.
-
Major Product: (CH3)3COH (tert-butyl alcohol). The product is racemic due to the planar carbocation intermediate.
Example 2: SN2 Reaction
CH3CH2Br + NaCN → ?
-
Functional Groups: Primary alkyl halide and cyanide ion (strong nucleophile).
-
Reaction Type: SN2 (favored by primary alkyl halides and strong nucleophiles).
-
Mechanism: The cyanide ion attacks the carbon atom bearing the bromine atom from the backside, leading to inversion of configuration.
-
Reaction Conditions: The solvent's nature influences the rate but doesn't drastically alter the outcome.
-
Major Product: CH3CH2CN (propanenitrile). The configuration at the carbon atom is inverted.
Example 3: E1 Reaction
(CH3)3CBr + Ethanol (heat) → ?
-
Functional Groups: Tertiary alkyl halide and ethanol (a weak base).
-
Reaction Type: E1 (favored by tertiary alkyl halides, heat, and a protic solvent).
-
Mechanism: The reaction proceeds through a carbocation intermediate, followed by proton removal by the solvent or the conjugate base of the solvent to form an alkene.
-
Reaction Conditions: Heat promotes E1.
-
Major Product: (CH3)2C=CH2 (2-methylpropene) (Zaitsev's rule predicts the most substituted alkene).
Example 4: E2 Reaction
CH3CH2Br + KOH (alcoholic) → ?
-
Functional Groups: Primary alkyl halide and strong base (potassium hydroxide).
-
Reaction Type: E2 (favored by strong bases).
-
Mechanism: A concerted mechanism involving simultaneous removal of the proton and the leaving group.
-
Reaction Conditions: Alcoholic KOH favors E2.
-
Major Product: CH2=CH2 (ethene).
Example 5: Electrophilic Addition
CH2=CH2 + HBr → ?
-
Functional Groups: Alkene and hydrogen bromide.
-
Reaction Type: Electrophilic addition.
-
Mechanism: The electrophile (H+) adds to the alkene, forming a carbocation intermediate. The bromide ion then attacks the carbocation.
-
Reaction Conditions: This reaction proceeds readily at room temperature.
-
Major Product: CH3CH2Br (bromoethane) (Markovnikov's rule dictates that the hydrogen adds to the carbon with more hydrogens).
Advanced Considerations: Beyond the Basics
This analysis focuses on simplified scenarios. In reality, many reactions involve competing pathways, side reactions, and complex stereochemical considerations. Factors such as steric hindrance, neighboring group participation, and the presence of multiple functional groups can significantly influence the outcome.
Steric Hindrance:
Bulky groups can hinder the approach of nucleophiles or bases, affecting reaction rates and selectivity.
Neighboring Group Participation:
The presence of a neighboring group with lone pairs can influence the reaction pathway, leading to unexpected products.
Multiple Functional Groups:
Reactions with molecules containing multiple functional groups can lead to complex mixtures of products. Identifying the most reactive functional group is essential.
Conclusion
Predicting the major organic product of a reaction requires a comprehensive understanding of reaction mechanisms, functional group transformations, and the impact of reaction conditions. This article provides a structured approach to tackling these problems. Remember, practice is crucial. Working through numerous examples will build your intuition and allow you to accurately predict the outcome of various organic reactions. The more you practice, the more confidently you will navigate the complexities of organic chemistry. By mastering these principles, you'll be well-equipped to successfully tackle more complex scenarios and become a proficient organic chemist.
Latest Posts
Latest Posts
-
Decide Whether These Proposed Lewis Structures Are Reasonable
Mar 20, 2025
-
Cherokee Incorporated Is A Merchandiser That Provided The Following Information
Mar 20, 2025
-
Which Of The Following Is Not A Function Of Blood
Mar 20, 2025
-
Challenge Yourself 2 3 Spring Hills Community
Mar 20, 2025
-
All Of The Following Describe Blockchain
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Draw The Major Organic Product Of The Reaction Shown Below . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
