Decide Whether These Proposed Lewis Structures Are Reasonable.
Holbox
Mar 20, 2025 · 6 min read
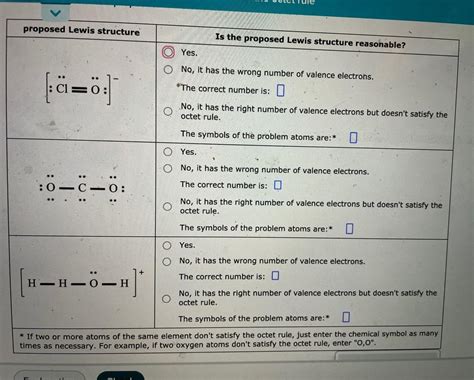
Table of Contents
Deciding Whether Proposed Lewis Structures Are Reasonable: A Comprehensive Guide
Lewis structures, also known as Lewis dot diagrams, are crucial for understanding chemical bonding and molecular geometry. They represent the valence electrons of atoms within a molecule, showing how these electrons are arranged to form bonds and lone pairs. However, not all proposed Lewis structures are valid or even reasonable. This article delves deep into the criteria for evaluating the reasonableness of a Lewis structure, providing you with a robust understanding of this fundamental concept in chemistry.
The Fundamental Rules of Lewis Structures
Before evaluating proposed structures, it's essential to understand the basic rules governing their construction. These rules help ensure a structure accurately reflects the molecule's electronic configuration:
1. Valence Electrons: The Building Blocks
The foundation of any Lewis structure lies in correctly counting the valence electrons of each atom involved. Valence electrons are the electrons in the outermost shell, and they're the ones participating in bonding. Knowing the group number of an element in the periodic table directly tells us its number of valence electrons. For example, carbon (Group 14) has four valence electrons, oxygen (Group 16) has six, and hydrogen (Group 1) has one.
2. Octet Rule (and Exceptions): The Stability Goal
The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable configuration with eight electrons in their outermost shell, mimicking the electron arrangement of noble gases. This configuration provides maximum stability. However, there are exceptions to the octet rule:
- Elements with fewer than eight electrons: Hydrogen and helium only need two electrons (duet rule) for stability. Boron and beryllium often have fewer than eight electrons in their compounds.
- Elements with more than eight electrons: Elements in the third period and beyond (like phosphorus, sulfur, and chlorine) can expand their octet and accommodate more than eight valence electrons. This is due to the availability of empty d-orbitals.
3. Formal Charge: Assessing Electron Distribution
Formal charge is a tool used to determine the most reasonable Lewis structure among multiple possibilities. It's the hypothetical charge assigned to an atom in a molecule, assuming that electrons in bonds are equally shared between the atoms. A lower formal charge on atoms usually indicates a more stable structure. The formal charge is calculated using the following formula:
Formal Charge = (Valence Electrons) - (Non-bonding Electrons) - (1/2 * Bonding Electrons)
4. Resonance Structures: Delocalized Electrons
Sometimes, a single Lewis structure isn't sufficient to represent a molecule accurately. In such cases, resonance structures are used. Resonance structures are different Lewis structures that can be drawn for the same molecule, differing only in the placement of electrons. The actual molecule is a hybrid of these resonance structures, with the electrons delocalized over multiple atoms.
Evaluating the Reasonableness of Proposed Lewis Structures
Now, let's delve into the criteria for assessing whether a proposed Lewis structure is reasonable:
1. Correct Valence Electron Count
The most fundamental check is verifying if the structure uses the correct number of valence electrons. Carefully count the valence electrons from each atom and compare it to the total number of electrons in the proposed structure. Discrepancies immediately indicate an error.
2. Octet Rule Fulfillment (with Exceptions)
Check if each atom (except hydrogen and helium) has achieved an octet (or duet for hydrogen and helium). Note the exceptions to the octet rule, particularly for elements in the third period and beyond that can exceed an octet.
3. Minimization of Formal Charges
Analyze the formal charges on each atom in the proposed structure. A structure with minimal formal charges (ideally zero) is generally more reasonable and stable than one with large formal charges. If formal charges are present, strive to have negative formal charges on the more electronegative atoms.
4. Resonance Structures (if applicable)
If multiple Lewis structures can be drawn for a molecule, consider resonance structures. The true structure is a resonance hybrid, reflecting the delocalization of electrons. A combination of resonance structures often provides a better representation of the actual molecule's electronic distribution than any single structure.
5. Electronegativity Considerations
Electronegativity, the tendency of an atom to attract electrons towards itself in a bond, plays a role. In general, less electronegative atoms should have positive formal charges while more electronegative atoms should have negative formal charges (if any).
6. Bond Lengths and Bond Orders
While Lewis structures don't explicitly show bond lengths, they can indicate bond order (the number of bonds between two atoms). Higher bond orders generally indicate shorter and stronger bonds. Compare the predicted bond orders from the Lewis structure to known experimental data, where available.
7. Molecular Geometry Predictions
Lewis structures can be used to predict the basic molecular geometry using the VSEPR (Valence Shell Electron Pair Repulsion) theory. While Lewis structures don't directly show 3D shape, they provide the basis for VSEPR predictions. Compare any predicted geometry with known experimental data to assess the structure's reasonableness.
Examples of Evaluating Lewis Structures
Let's illustrate the process with examples:
Example 1: CO₂ (Carbon Dioxide)
A proposed Lewis structure shows carbon with a triple bond to one oxygen and a single bond to the other oxygen. This structure assigns a +1 formal charge to carbon and a -1 formal charge to one oxygen atom, and a 0 formal charge to the other.
Evaluation: This structure is unreasonable. The formal charges are not minimized. A more reasonable structure would show carbon double-bonded to both oxygen atoms, resulting in zero formal charge on all atoms, adhering to the octet rule for all atoms.
Example 2: SO₃ (Sulfur Trioxide)
A proposed structure shows sulfur with single bonds to all three oxygen atoms. This results in sulfur having a +2 formal charge and each oxygen having a -1 formal charge.
Evaluation: This is less reasonable than a structure involving resonance, with sulfur double-bonded to two oxygens and single-bonded to one oxygen. While formal charges are present, they are lower than in the previous structure, and the resonance among these structures better reflects the delocalized electrons.
Example 3: PF₅ (Phosphorus Pentafluoride)
A proposed Lewis structure shows phosphorus with five single bonds to five fluorine atoms. This results in phosphorus having 10 electrons in its valence shell, exceeding the octet.
Evaluation: This structure is reasonable. Phosphorus, being in the third period, can expand its octet.
Conclusion: A Holistic Approach
Determining the reasonableness of a Lewis structure is a multi-step process. No single criterion is definitive. A holistic approach, considering the valence electron count, octet rule, formal charges, resonance, electronegativity, predicted bond orders, and molecular geometry (where applicable), provides a comprehensive evaluation of any proposed structure's validity. By applying these principles consistently, you can confidently assess Lewis structures and gain a deeper understanding of chemical bonding. This comprehensive approach is crucial in accurately representing molecular structures and understanding their properties. Remember that the most reasonable structure is the one that best explains the molecule's observed properties, including experimental data when available.
Latest Posts
Latest Posts
-
The Mercalli Scale Is A Scale From
Mar 20, 2025
-
The Influence Of Interest Groups Through The Courts Occurs Through
Mar 20, 2025
-
An Example Of An Intermediate Good Would Be
Mar 20, 2025
-
How Do Smartphones Achieve Images With High Dynamic Range
Mar 20, 2025
-
Based On Values In Cells A51 A55
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Decide Whether These Proposed Lewis Structures Are Reasonable. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
