Draw An Outer Electron Box Diagram For A Cation
Holbox
Mar 15, 2025 · 5 min read
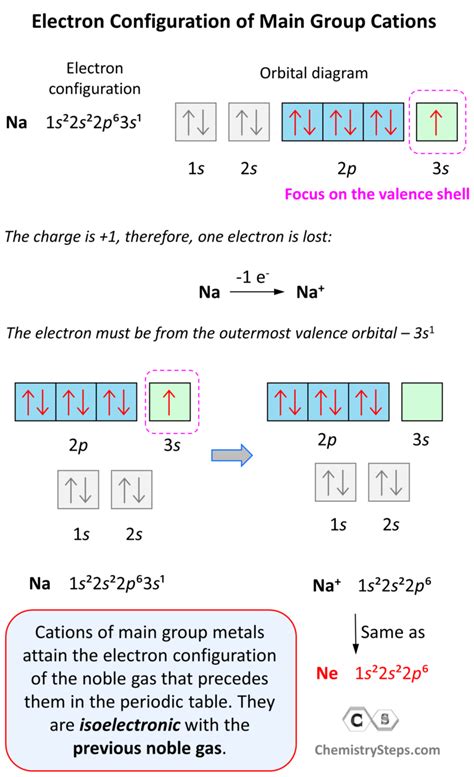
Table of Contents
Drawing Outer Electron Box Diagrams for Cations: A Comprehensive Guide
Drawing electron box diagrams, also known as orbital box diagrams or electron configuration diagrams, is a fundamental skill in chemistry. These diagrams visually represent the arrangement of electrons within an atom's orbitals, providing a clear understanding of an atom's electronic structure and its chemical behavior. This guide focuses specifically on creating outer electron box diagrams for cations, positively charged ions formed when an atom loses one or more electrons.
Understanding Electron Configurations and Orbitals
Before diving into drawing diagrams for cations, let's refresh some key concepts:
-
Electron Configuration: This describes the arrangement of electrons in an atom's orbitals. It follows specific rules, including the Aufbau principle (electrons fill lower energy levels first), Hund's rule (electrons fill orbitals individually before pairing up), and the Pauli exclusion principle (each orbital can hold a maximum of two electrons with opposite spins).
-
Orbitals: These are regions within an atom where there's a high probability of finding an electron. Different orbitals have different shapes and energy levels. The principal energy levels (n = 1, 2, 3, etc.) contain sublevels (s, p, d, f), each capable of holding a specific number of electrons.
- s orbitals: Hold a maximum of 2 electrons.
- p orbitals: Hold a maximum of 6 electrons (3 orbitals, each holding 2 electrons).
- d orbitals: Hold a maximum of 10 electrons (5 orbitals, each holding 2 electrons).
- f orbitals: Hold a maximum of 14 electrons (7 orbitals, each holding 2 electrons).
Creating Outer Electron Box Diagrams for Neutral Atoms
To understand cation diagrams, we must first master neutral atom diagrams. Let's illustrate with an example: Oxygen (O). Oxygen has an atomic number of 8, meaning it has 8 electrons. Its electron configuration is 1s²2s²2p⁴.
The outer electron box diagram would look like this:
1s: ↑↓
2s: ↑↓
2p: ↑↓ ↑ ↑ (Note: There are three 2p orbitals, each represented by a box.)
Each arrow represents an electron, and the up and down arrows indicate opposite spins.
Forming Cations and Modifying Electron Box Diagrams
A cation forms when a neutral atom loses one or more valence electrons (electrons in the outermost shell). The number of electrons lost determines the cation's charge. For example:
-
Sodium (Na) forming Na⁺: Sodium has an electron configuration of 1s²2s²2p⁶3s¹. To become a +1 cation (Na⁺), it loses one electron from its 3s orbital.
-
Magnesium (Mg) forming Mg²⁺: Magnesium has an electron configuration of 1s²2s²2p⁶3s². To become a +2 cation (Mg²⁺), it loses two electrons, both from its 3s orbital.
-
Aluminum (Al) forming Al³⁺: Aluminum has an electron configuration of 1s²2s²2p⁶3s²3p¹. To become a +3 cation (Al³⁺), it loses three electrons: two from the 3s orbital and one from the 3p orbital.
Drawing Outer Electron Box Diagrams for Cations: Step-by-Step
Let's illustrate the process with several examples:
1. Sodium Cation (Na⁺):
-
Step 1: Determine the electron configuration of the neutral atom. Sodium (Na) has 11 electrons: 1s²2s²2p⁶3s¹.
-
Step 2: Identify the valence electrons. The valence electrons are those in the outermost shell (n=3 in this case). Sodium has one valence electron in the 3s orbital.
-
Step 3: Determine the number of electrons lost. Sodium loses one electron to become Na⁺.
-
Step 4: Draw the outer electron box diagram for the cation. Since Sodium lost one electron from its 3s orbital, the outer electron box diagram for Na⁺ will only show the filled inner shells and the empty 3s orbital.
3s: (empty)
2. Magnesium Cation (Mg²⁺):
-
Step 1: Determine the electron configuration of the neutral atom. Magnesium (Mg) has 12 electrons: 1s²2s²2p⁶3s².
-
Step 2: Identify the valence electrons. Magnesium has two valence electrons in the 3s orbital.
-
Step 3: Determine the number of electrons lost. Magnesium loses two electrons to become Mg²⁺.
-
Step 4: Draw the outer electron box diagram for the cation. Both electrons from the 3s orbital are removed.
3s: (empty)
3. Aluminum Cation (Al³⁺):
-
Step 1: Determine the electron configuration of the neutral atom. Aluminum (Al) has 13 electrons: 1s²2s²2p⁶3s²3p¹.
-
Step 2: Identify the valence electrons. Aluminum has three valence electrons: two in the 3s orbital and one in the 3p orbital.
-
Step 3: Determine the number of electrons lost. Aluminum loses three electrons to become Al³⁺.
-
Step 4: Draw the outer electron box diagram for the cation. All three valence electrons are removed.
3s: (empty)
3p: (empty)
4. Transition Metal Cations: A More Complex Scenario
Transition metals often form multiple cations. Consider Iron (Fe):
-
Fe²⁺: Iron loses two electrons, typically from the 4s orbital first, then one from the 3d orbital. Its electron configuration would be [Ar]3d⁶. The outer electron box diagram would show the 3d orbitals partially filled.
-
Fe³⁺: Iron loses three electrons, typically two from the 4s and one from the 3d orbital, resulting in the electron configuration [Ar]3d⁵. Again, the outer electron box diagram will reflect the occupancy of the 3d orbitals.
The exact orbital order from which electrons are removed can be complex and may depend on other factors, such as ligand field effects. However, the fundamental principle of removing valence electrons remains the same.
Importance of Outer Electron Box Diagrams for Cations
Understanding the outer electron box diagrams for cations is crucial for several reasons:
-
Predicting Chemical Behavior: The arrangement of electrons in the outermost shell determines an ion's reactivity and bonding behavior. Cations, with their electron deficiency, readily participate in ionic bonding with anions.
-
Understanding Ionic Compounds: Drawing these diagrams helps visualize how cations and anions interact to form stable ionic compounds.
-
Explaining Properties of Compounds: The electronic structure affects properties such as melting point, boiling point, and solubility.
-
Solving Chemical Problems: These diagrams are essential tools for solving stoichiometry problems, predicting reaction products, and understanding chemical processes.
Conclusion
Mastering the art of drawing outer electron box diagrams for cations is a fundamental step towards developing a strong understanding of chemical bonding, reactivity, and the properties of ionic compounds. The step-by-step process outlined in this guide, along with numerous examples, provides a solid foundation for this essential chemical skill. Remember to always start with the electron configuration of the neutral atom, then systematically remove electrons to form the cation, reflecting the change accurately in your final diagram. Consistent practice will enhance your proficiency and allow you to confidently tackle more complex scenarios.
Latest Posts
Latest Posts
-
The Waiting Times Between A Subway Departure
Mar 15, 2025
-
A Improvement In Production Technology Will Shift The
Mar 15, 2025
-
Select The Nmr Spectrum That Corresponds Best To P Anisidine
Mar 15, 2025
-
An Increase In The Quantity Supplied Suggests A
Mar 15, 2025
-
Libreoffice Is An Example Of Which Type Of Software
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Draw An Outer Electron Box Diagram For A Cation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
