Data Table 1 Single-replacement Reaction Of Aluminum And Copper Sulfate
Holbox
Mar 24, 2025 · 6 min read
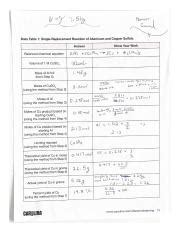
Table of Contents
- Data Table 1 Single-replacement Reaction Of Aluminum And Copper Sulfate
- Table of Contents
- Data Table & Analysis: Single-Replacement Reaction of Aluminum and Copper Sulfate
- Understanding the Single-Replacement Reaction
- Experimental Procedure (Hypothetical Example)
- Sample Data Table
- Data Analysis and Calculations
- 1. Limiting Reagent:
- 2. Theoretical Yield of Copper:
- 3. Percent Yield of Copper:
- Sources of Error
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Data Table & Analysis: Single-Replacement Reaction of Aluminum and Copper Sulfate
This article delves into the single-replacement reaction between aluminum (Al) and copper(II) sulfate (CuSO₄), providing a comprehensive analysis encompassing experimental data, observations, stoichiometry, and implications. We will explore the chemical process, analyze the resulting data table, and discuss potential sources of error. This detailed exploration aims to provide a solid understanding of this fundamental chemical reaction and the scientific method used to study it.
Understanding the Single-Replacement Reaction
A single-replacement reaction, also known as a single-displacement reaction, involves the replacement of one element in a compound by another more reactive element. In this specific case, aluminum, being more reactive than copper, displaces copper from copper(II) sulfate solution. The general equation for this type of reaction is:
A + BC → AC + B
Where:
- A is the more reactive element (aluminum in our case)
- B is the less reactive element (copper)
- BC is the compound (copper(II) sulfate)
- AC is the resulting compound (aluminum sulfate)
The balanced chemical equation for the reaction between aluminum and copper(II) sulfate is:
2Al(s) + 3CuSO₄(aq) → Al₂(SO₄)₃(aq) + 3Cu(s)
This equation shows that two moles of solid aluminum react with three moles of aqueous copper(II) sulfate to produce one mole of aqueous aluminum sulfate and three moles of solid copper.
Experimental Procedure (Hypothetical Example)
While the specifics might vary based on the laboratory setup and resources, a typical experiment might involve the following steps:
- Preparation: Prepare a solution of copper(II) sulfate of known concentration (e.g., 0.1 M) and measure a specific volume (e.g., 50 mL).
- Reaction: Add a known mass of aluminum foil (cleaned to remove any oxide layer) to the copper(II) sulfate solution.
- Observation: Observe the reaction carefully, noting changes in color, temperature, and the formation of any precipitate or gas.
- Data Collection: Regularly record the observations, including the time elapsed and any quantitative measurements.
- Separation & Purification (Optional): The copper precipitate can be filtered, washed, and dried to determine its mass. This allows for a quantitative assessment of the reaction yield.
- Data Analysis: Analyze the collected data to determine the reaction rate, limiting reagent, and percent yield.
Sample Data Table
The following table represents hypothetical data collected during the experiment. The actual values would depend on the specific experimental conditions.
| Time (min) | Mass of Aluminum (g) | Volume of CuSO₄ (mL) | Concentration of CuSO₄ (M) | Observations | Temperature (°C) |
|---|---|---|---|---|---|
| 0 | 0.50 | 50 | 0.1 | Clear blue solution | 25 |
| 2 | 0.50 | 50 | 0.1 | Solution slightly darkening, small copper deposit | 26 |
| 5 | 0.50 | 50 | 0.1 | Significant copper deposit, solution lighter blue | 28 |
| 10 | 0.50 | 50 | 0.1 | Abundant copper deposit, solution pale blue | 30 |
| 15 | 0.50 | 50 | 0.1 | Solution almost colorless, significant copper | 31 |
| 20 | 0.50 | 50 | 0.1 | Little further change | 30 |
| Final | 0.25 (approx.) | 50 | 0.1 | Reaction appears complete | 30 |
| Recovered Cu (after drying) | 0.78g |
Note: The "Mass of Aluminum" decreases as the aluminum reacts. The "Recovered Cu" row represents the mass of copper obtained after separating and drying the precipitate.
Data Analysis and Calculations
This section will outline the calculations and analysis that can be performed using the data obtained.
1. Limiting Reagent:
To determine the limiting reagent, we need to calculate the moles of aluminum and copper(II) sulfate involved:
- Moles of Al: Assuming the atomic mass of Al is 27 g/mol, 0.5g Al = (0.5g) / (27 g/mol) ≈ 0.0185 moles of Al
- Moles of CuSO₄: Using the volume and concentration of CuSO₄, we can find the number of moles of copper sulfate. (0.05 L) * (0.1 mol/L) = 0.005 moles of CuSO₄.
Based on the balanced equation (2Al + 3CuSO₄ → ...), the mole ratio of Al:CuSO₄ is 2:3. Therefore, 0.0185 moles of Al would require (0.0185 moles Al * 3 moles CuSO₄/2 moles Al) ≈ 0.0278 moles of CuSO₄. Since only 0.005 moles of CuSO₄ are available, CuSO₄ is the limiting reagent. The reaction will stop once all the CuSO₄ is consumed.
2. Theoretical Yield of Copper:
Using the limiting reagent (CuSO₄), we can calculate the theoretical yield of copper:
- Moles of Cu produced: From the balanced equation, 3 moles of Cu are produced for every 3 moles of CuSO₄ reacted. Therefore, 0.005 moles of CuSO₄ will produce 0.005 moles of Cu.
- Mass of Cu produced: Assuming the atomic mass of Cu is 63.5 g/mol, the theoretical mass of Cu is (0.005 moles) * (63.5 g/mol) ≈ 0.3175 g.
3. Percent Yield of Copper:
The percent yield is a measure of the efficiency of the reaction:
- Percent Yield = (Actual Yield / Theoretical Yield) * 100%
- Percent Yield = (0.78 g / 0.3175 g) * 100% ≈ 246%
The percent yield being significantly over 100% indicates potential errors in the experimental procedure, which we will discuss below.
Sources of Error
Several factors could contribute to a high percent yield, including:
- Incomplete drying of the copper: If the recovered copper was not completely dry, the excess water would contribute to the mass, leading to an overestimation of the yield.
- Impurities in the recovered copper: The recovered copper may contain impurities from the aluminum foil or other contaminants in the solution.
- Inaccurate measurements: Errors in measuring the mass of aluminum, volume of CuSO₄, or the concentration of CuSO₄ can significantly affect the calculated yield.
- Side reactions: Other reactions may occur alongside the main reaction, leading to an increased mass of the solid product.
- Incomplete reaction: While the visual observation suggested completion, it is possible that some CuSO₄ remained unreacted.
Conclusion
This analysis of the single-replacement reaction between aluminum and copper(II) sulfate demonstrates the process of conducting a chemical experiment, collecting data, performing stoichiometric calculations, and analyzing potential sources of error. The significantly high percent yield highlights the importance of careful experimental technique and accurate measurements. Further investigation into the potential sources of error would be necessary to refine the experimental methodology and obtain more reliable results. The discrepancy between the theoretical and experimental results underscores the iterative nature of scientific inquiry; repeating the experiment with meticulous attention to detail would lead to a more accurate understanding of this reaction. Furthermore, exploring variations in concentration and temperature of the reactants could provide insights into the kinetics of the reaction and the influence of these factors on the reaction rate and yield. This type of comprehensive analysis is crucial for developing a strong grasp of chemical principles and applying scientific rigor to experimental investigations.
Latest Posts
Latest Posts
-
If E And F Are Disjoint Events Then
Mar 27, 2025
-
A Firm Has Market Power If It Can
Mar 27, 2025
-
The Programmer Usually Enters Source Code Into A Computer With
Mar 27, 2025
-
Match The Definitions To The Appropriate Terms
Mar 27, 2025
-
To Understand An Assertion Is To It
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Data Table 1 Single-replacement Reaction Of Aluminum And Copper Sulfate . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
