Conversion Of 2-methyl-2-butene Into A Secondary Alkyl Halide
Holbox
Mar 24, 2025 · 7 min read
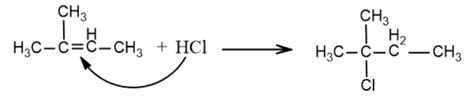
Table of Contents
- Conversion Of 2-methyl-2-butene Into A Secondary Alkyl Halide
- Table of Contents
- Conversion of 2-Methyl-2-butene into a Secondary Alkyl Halide: A Comprehensive Guide
- Understanding the Starting Material: 2-Methyl-2-butene
- The Challenge: Achieving Secondary Halide Formation
- Strategies for Conversion to a Secondary Alkyl Halide
- 1. Hydroboration-Oxidation followed by Substitution
- 2. Allylic Bromination followed by SN2 Reaction
- 3. Epoxidation followed by Ring Opening
- Factors Affecting Regioselectivity
- Analytical Techniques for Product Identification
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Conversion of 2-Methyl-2-butene into a Secondary Alkyl Halide: A Comprehensive Guide
The conversion of 2-methyl-2-butene, a tertiary alkene, into a secondary alkyl halide presents a fascinating challenge in organic chemistry. This transformation requires careful consideration of reaction mechanisms and regioselectivity to achieve the desired product. This detailed guide will explore various methods, focusing on the intricacies of achieving this specific conversion, including reaction conditions, mechanisms, and potential challenges. We will delve into the nuances of electrophilic addition and the importance of controlling carbocation rearrangements to obtain the secondary halide.
Understanding the Starting Material: 2-Methyl-2-butene
2-Methyl-2-butene is a branched, unsaturated hydrocarbon with the chemical formula C₅H₁₀. Its structure features a double bond between carbon atoms 2 and 3, with a methyl group attached to carbon 2. This specific arrangement is crucial in understanding the challenges of converting it into a secondary alkyl halide. The double bond's position and the presence of the methyl group influence the reactivity and stability of intermediate carbocations formed during the reaction.
The Challenge: Achieving Secondary Halide Formation
The direct addition of a hydrogen halide (HX, where X represents a halogen like chlorine or bromine) to 2-methyl-2-butene would typically yield a tertiary alkyl halide. This is because the initial carbocation intermediate formed is a tertiary carbocation, which is the most stable carbocation due to hyperconjugation and inductive effects. The tertiary carbocation then readily reacts with the halide ion (X⁻) to form the tertiary halide. To obtain a secondary halide, we must employ strategies that circumvent the formation of the tertiary carbocation or manipulate the reaction pathway to favor the secondary product.
Strategies for Conversion to a Secondary Alkyl Halide
Several approaches can be employed to steer the reaction towards the formation of a secondary alkyl halide from 2-methyl-2-butene. These strategies primarily focus on manipulating the reaction conditions or employing alternative reagents to influence the reaction mechanism.
1. Hydroboration-Oxidation followed by Substitution
This multi-step approach offers a viable route to a secondary alkyl halide.
Step 1: Hydroboration
Hydroboration of 2-methyl-2-butene using borane (BH₃) or a borane derivative (like 9-borabicyclo[3.3.1]nonane, or 9-BBN) proceeds via an anti-Markovnikov addition. The boron atom adds to the less substituted carbon atom (carbon 3), resulting in a secondary organoborane. This step avoids the formation of a carbocation intermediate. The mechanism involves a concerted addition of the boron atom and a hydrogen atom across the double bond.
Step 2: Oxidation
The organoborane intermediate is then oxidized using hydrogen peroxide (H₂O₂) in the presence of a base (like sodium hydroxide, NaOH). This oxidation step replaces the boron atom with a hydroxyl group (-OH), forming a secondary alcohol, 3-methyl-2-butanol. The oxidation is a syn addition, meaning the hydroxyl group adds to the same side as the boron atom.
Step 3: Conversion to Alkyl Halide
The secondary alcohol, 3-methyl-2-butanol, can then be converted to the corresponding secondary alkyl halide using a suitable reagent. Common methods include:
-
Reaction with hydrogen halides (HX): Reaction with concentrated hydrohalic acids (HCl, HBr, or HI) in the presence of a catalyst like zinc chloride (ZnCl₂) can lead to an SN1 or SN2 reaction, depending on the conditions. The SN2 reaction is favored if the reaction is carried out under anhydrous conditions with a strong acid.
-
Reaction with thionyl chloride (SOCl₂): This reagent is particularly useful for converting alcohols to alkyl chlorides. The reaction proceeds via an SN2 mechanism and often requires a solvent like pyridine.
-
Reaction with phosphorus tribromide (PBr₃): This reagent is used for converting alcohols to alkyl bromides via an SN2 mechanism.
This hydroboration-oxidation-substitution sequence effectively avoids the formation of a tertiary carbocation and produces the desired secondary alkyl halide.
2. Allylic Bromination followed by SN2 Reaction
This strategy involves converting the alkene into an allylic halide first, and then transforming it into a secondary halide.
Step 1: Allylic Bromination
Treatment of 2-methyl-2-butene with N-bromosuccinimide (NBS) in the presence of light or a radical initiator like benzoyl peroxide will lead to allylic bromination. This reaction involves the formation of an allylic radical, which is then stabilized through resonance. The reaction predominantly occurs at the allylic position, leading to a mixture of allylic bromides, including 3-bromo-2-methyl-2-butene and other isomers.
Step 2: SN2 Reaction
The mixture of allylic bromides can be subjected to an SN2 reaction with a nucleophile to replace the bromine atom. The selection of the nucleophile and reaction conditions are crucial for achieving selectivity towards the secondary halide. Careful control of the reaction conditions, including temperature and choice of solvent, is critical to minimize the formation of tertiary products via potential rearrangements. A strong nucleophile under appropriate conditions could favor the SN2 pathway, leading to the substitution of the allylic bromine to generate the secondary alkyl halide.
However, this pathway could be complex and might produce a mixture of products due to the potential for rearrangements and competing reaction pathways.
3. Epoxidation followed by Ring Opening
This approach involves creating an epoxide intermediate and then selectively opening the ring to obtain the secondary halide.
Step 1: Epoxidation
Epoxidation of 2-methyl-2-butene using a peroxyacid like meta-chloroperoxybenzoic acid (mCPBA) yields an epoxide. This reaction adds an oxygen atom across the double bond, forming a three-membered ring.
Step 2: Regioselective Ring Opening
Regioselective ring opening of the epoxide can be achieved using a halide ion (X⁻) in the presence of an acid catalyst. The ring-opening reaction can be influenced by the choice of halide and reaction conditions. While not entirely straightforward to control regioselectivity in this case, under specific conditions (acid catalysis and careful control of the nucleophile), a secondary halide could potentially be favored. However, this might require a complex optimization of the reaction conditions. Stereochemical control is also important to consider in this approach.
Factors Affecting Regioselectivity
Regioselectivity, the preference for formation of one constitutional isomer over another, is a critical factor in the conversion of 2-methyl-2-butene into a secondary alkyl halide. Several factors influence regioselectivity:
-
Carbocation Stability: The stability of carbocation intermediates plays a major role in determining the product distribution. Tertiary carbocations are more stable than secondary carbocations, hence the tendency to form the tertiary halide.
-
Steric Hindrance: Steric hindrance can affect the approach of the nucleophile to the carbocation or the substrate, influencing the reaction pathway.
-
Reaction Conditions: Reaction temperature, solvent, and the concentration of reagents can all significantly influence the regioselectivity of the reaction.
-
Reagent Choice: The choice of reagent, particularly in the substitution step, plays a crucial role in determining the product distribution. For example, the use of specific reagents can favor SN1 or SN2 mechanisms, influencing the outcome.
Analytical Techniques for Product Identification
Confirmation of the successful conversion of 2-methyl-2-butene into the desired secondary alkyl halide requires employing appropriate analytical techniques. These include:
-
Nuclear Magnetic Resonance (NMR) Spectroscopy: ¹H and ¹³C NMR spectroscopy can be used to identify the structure of the product by analyzing the chemical shifts, coupling constants, and integration values. The presence of characteristic peaks for the secondary alkyl halide can be used for confirmation.
-
Gas Chromatography-Mass Spectrometry (GC-MS): GC-MS can be used for the separation and identification of the reaction products. The mass spectrum provides information about the molecular weight and fragmentation pattern of the compound.
-
Infrared (IR) Spectroscopy: IR spectroscopy can be used to confirm the presence of characteristic functional groups in the product molecule, such as C-X stretching vibrations for the alkyl halide.
Conclusion
The conversion of 2-methyl-2-butene into a secondary alkyl halide is not a straightforward process. It requires strategic manipulation of reaction pathways to overcome the inherent preference for the formation of the tertiary halide. Hydroboration-oxidation followed by substitution presents a reliable pathway to achieve this transformation by avoiding the formation of the tertiary carbocation intermediate altogether. However, the other methods, allylic bromination and epoxidation, while potentially applicable, require careful optimization and control of reaction conditions to minimize the formation of unwanted products and achieve the desired regioselectivity. Careful consideration of reaction mechanisms, regioselectivity, and analytical techniques are essential for the successful completion of this transformation. Further research and optimization might be necessary to refine the existing methods and develop new, more efficient approaches for this important conversion in organic synthesis.
Latest Posts
Latest Posts
-
Paraphrasing Another Authors Paragraph By Substituting
Mar 29, 2025
-
You Then Ping A Website With An Ip Address
Mar 29, 2025
-
Label The Following Diagram Of Earths Layers
Mar 29, 2025
-
Grazing Animals Such As Deer Are
Mar 29, 2025
-
Personnel Services Contracts Are Authorized By The Government When
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Conversion Of 2-methyl-2-butene Into A Secondary Alkyl Halide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
