Continuing Review Of An Approved And Ongoing Study Posing
Holbox
Mar 22, 2025 · 6 min read
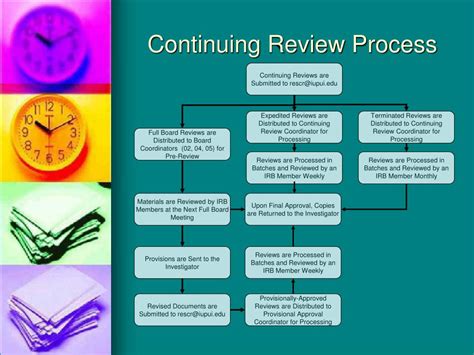
Table of Contents
Continuing Review of an Approved and Ongoing Study: A Comprehensive Guide
The ethical conduct of research involving human subjects is paramount. Once a study receives initial approval from an Institutional Review Board (IRB) or ethics committee, the process doesn't end. Ongoing research requires consistent oversight and monitoring through a process known as continuing review. This article delves into the intricacies of continuing review, explaining its purpose, requirements, and best practices to ensure the ethical and scientific integrity of your study.
Understanding the Purpose of Continuing Review
Continuing review is a systematic process designed to ensure the continued protection of human subjects participating in ongoing research. It's not simply a formality; it's a critical safeguard against potential risks and a mechanism for adapting the study based on evolving circumstances. The primary goals include:
- Monitoring participant safety and well-being: Regular review allows the IRB to assess whether participants are experiencing any unforeseen adverse events or risks.
- Evaluating the study's scientific integrity: Continuing review assesses whether the study is progressing as planned, producing meaningful data, and adhering to its methodology.
- Assessing the continued relevance and feasibility of the study: Changes in the scientific landscape, new research findings, or evolving societal norms may necessitate adjustments to the study protocol.
- Ensuring compliance with regulations and ethical guidelines: Continuing review helps maintain compliance with relevant federal regulations, institutional policies, and ethical principles.
Key Differences from Initial Review
While both initial and continuing reviews assess the ethical and scientific merits of a study, they differ significantly in focus and scope. Initial review is a comprehensive assessment of the entire research protocol, including methodology, risks and benefits, informed consent procedures, and participant selection criteria. Continuing review, on the other hand, focuses on the ongoing conduct of the research, primarily evaluating whether the study continues to meet ethical standards and whether the initial risks and benefits assessment remains accurate.
Frequency of Continuing Review
The frequency of continuing review is determined by the IRB, but it's typically conducted annually for most studies. However, more frequent reviews may be required depending on several factors, including:
- The level of risk to participants: Studies involving higher risks will generally require more frequent review.
- The duration of the study: Longer studies may require more frequent reviews to account for potential changes over time.
- The nature of the data collected: Studies involving sensitive data may necessitate more rigorous monitoring.
- Significant changes to the study protocol: Any substantial modifications to the research protocol will trigger an expedited review.
Circumstances Requiring Expedited Review
Even between scheduled continuing reviews, specific events can necessitate an expedited review. These include:
- Adverse events or unexpected problems: Any serious adverse events, unexpected problems, or breaches in protocol must be reported immediately to the IRB and may trigger an expedited review.
- Significant protocol deviations: Any deviations from the approved protocol, especially those affecting participant safety or the validity of the data, require immediate reporting and review.
- Changes in investigator personnel: Changes in principal investigators or key personnel involved in the study may require an expedited review.
- Emergence of new information: New scientific findings or ethical considerations could necessitate a reassessment of the study's risks and benefits.
Components of a Continuing Review Application
The continuing review application typically includes the following:
- Progress report: A detailed summary of the study's progress, including enrollment numbers, data collection status, and any significant findings.
- Adverse event reports: A comprehensive report detailing any adverse events experienced by participants, including the nature, severity, and management of these events.
- Protocol amendments: Documentation of any changes made to the original research protocol, along with justifications for these modifications.
- Recruitment and enrollment data: An updated summary of recruitment strategies and enrollment numbers.
- Informed consent process update: Confirmation that informed consent procedures are being followed consistently and that participants are adequately informed about the study's purpose, risks, and benefits.
- Data management and security: An explanation of how data is being stored, managed, and protected to ensure confidentiality and security.
- Continuing assurances of compliance: A statement confirming adherence to all relevant regulations, institutional policies, and ethical guidelines.
Best Practices for Continuing Review
- Proactive monitoring: Researchers should proactively monitor participant safety and well-being throughout the study.
- Maintaining accurate records: Meticulous record-keeping is crucial for demonstrating compliance and providing the IRB with necessary information.
- Open communication with the IRB: Researchers should maintain open communication with the IRB and promptly report any issues or changes.
- Regular meetings with the research team: Regular meetings among research personnel ensure consistent implementation of the protocol and facilitate early identification of potential problems.
- Regular review of data: Regularly analyzing data can aid in early detection of unexpected trends or results that may require changes to the protocol or additional safety measures.
Avoiding Common Pitfalls
- Delays in submission: Submitting continuing review applications late can lead to delays in the study and potential ethical concerns.
- Incomplete or inaccurate information: Providing incomplete or inaccurate information to the IRB can undermine the review process and compromise participant safety.
- Failure to report adverse events: Not reporting adverse events promptly can have serious consequences for participants and the reputation of the research team.
- Ignoring protocol deviations: Ignoring or downplaying protocol deviations can erode the integrity of the research and undermine the credibility of the study.
The Role of the IRB in Continuing Review
The IRB plays a crucial role in overseeing continuing review. They carefully evaluate the information provided by the researchers, assess the ongoing risks and benefits to participants, and ensure compliance with ethical guidelines. The IRB may:
- Approve the study to continue as is: If the study is progressing according to plan and meets ethical standards, the IRB will typically approve its continuation.
- Request modifications: The IRB may request modifications to the study protocol to address any identified concerns or risks.
- Suspend or terminate the study: In cases of serious ethical violations or significant risks to participants, the IRB may suspend or terminate the study.
Conclusion: A Continuous Commitment to Ethical Research
Continuing review is not a mere administrative hurdle; it is an essential component of ensuring ethical and scientific rigor in research involving human subjects. By diligently adhering to the requirements and best practices outlined in this guide, researchers can contribute to a culture of responsible research, protect the rights and well-being of participants, and maintain the highest standards of scientific integrity. Remember, a commitment to ethical research is a continuous process, extending far beyond the initial approval phase. Regular, thorough continuing review is a vital aspect of that ongoing commitment. The proactive approach to this process guarantees not only the ethical and scientific soundness of the research, but also protects the well-being of the participants and upholds the researcher's credibility. Careful attention to detail and consistent communication with the IRB are key elements in successfully navigating this crucial step of the research process.
Latest Posts
Latest Posts
-
The Allele For Black Noses In Wolves
Mar 22, 2025
-
Why Do Companies Use Online Collaborative Productivity Software
Mar 22, 2025
-
Algebra Concepts And Connections Unit 1 Answer Key
Mar 22, 2025
-
A Ribbon Diagram Of A Zinc Metallo Beta Lactamase Protein Is Shown
Mar 22, 2025
-
The Most Likely Cause Of Bedding In This Image Is
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Continuing Review Of An Approved And Ongoing Study Posing . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
