Complete The Synthesis Below By Selecting Or Drawing The Reagents.
Holbox
Mar 19, 2025 · 5 min read
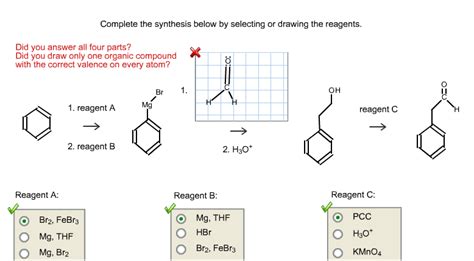
Table of Contents
Completing Organic Synthesis: A Comprehensive Guide to Reagent Selection and Reaction Design
Organic synthesis, the art and science of constructing complex molecules from simpler building blocks, is a cornerstone of chemistry. Successfully completing a synthesis requires a deep understanding of reaction mechanisms, functional group transformations, and, critically, the strategic selection of appropriate reagents. This article will delve into the process of completing a synthesis, focusing on the crucial step of reagent selection, providing examples and strategies for tackling various synthetic challenges. We'll explore how to analyze a target molecule, devise a retrosynthetic plan, and choose the right reagents to achieve each step effectively.
Understanding the Synthesis Problem: Retrosynthetic Analysis
Before even considering reagents, you must thoroughly understand the target molecule. This involves a systematic process called retrosynthetic analysis, where you work backward from the product to identify simpler precursors. This process often involves disconnecting bonds, identifying key functional groups, and considering potential reaction pathways.
Key questions to ask during retrosynthetic analysis:
- What are the key functional groups in the target molecule? Identifying these is crucial, as they dictate the types of reactions needed.
- What are the most readily available starting materials? Choosing commercially available starting materials simplifies the synthesis and reduces cost.
- What are the potential disconnections? This involves identifying bonds that can be broken to yield simpler fragments. Consider the feasibility and selectivity of each disconnection.
- What are the potential side reactions? Anticipating side reactions is crucial for optimizing yield and purity.
- What reaction conditions are compatible with the functional groups present? Certain reagents and conditions may interfere with existing functional groups.
Reagent Selection: The Heart of Organic Synthesis
Once the retrosynthetic pathway is established, the next crucial step is selecting the appropriate reagents for each transformation. The choice of reagent is influenced by several factors:
- Reaction Specificity: The reagent should selectively transform the desired functional group without affecting other functional groups present in the molecule. Regioselectivity and stereoselectivity are critical considerations.
- Reaction Efficiency: The reagent should provide a high yield of the desired product, minimizing the formation of side products.
- Reaction Conditions: The reaction conditions (temperature, solvent, pressure) should be compatible with both the reagent and the substrate.
- Cost and Availability: Cost-effectiveness and ease of access are practical considerations in reagent selection.
- Safety: The reagent should be safe to handle and environmentally benign.
Examples of Reagent Selection and Common Transformations
Let's explore some common organic transformations and the reagents used to achieve them:
1. Oxidation Reactions:
- Converting primary alcohols to aldehydes: Pyridinium chlorochromate (PCC) is a mild oxidizing agent that selectively oxidizes primary alcohols to aldehydes without over-oxidation to carboxylic acids. Other options include Dess-Martin periodinane (DMP) and Swern oxidation.
- Converting primary alcohols to carboxylic acids: Stronger oxidizing agents like potassium permanganate (KMnO4) or chromic acid (H2CrO4) are required for this transformation.
- Converting secondary alcohols to ketones: PCC, DMP, and Swern oxidation are also effective for this transformation.
2. Reduction Reactions:
- Reducing ketones and aldehydes to alcohols: Sodium borohydride (NaBH4) is a common reducing agent for this transformation. Lithium aluminum hydride (LiAlH4) is a more powerful reducing agent but is more reactive and requires anhydrous conditions.
- Reducing esters to alcohols: LiAlH4 is typically used for this transformation.
- Reducing alkynes to alkenes: Hydrogenation using a metal catalyst (e.g., Pd/C, Pt/C) is a common method. Lindlar's catalyst allows for the selective reduction of alkynes to cis-alkenes.
3. Alkylation Reactions:
- Alkylating Grignard reagents: Grignard reagents (RMgX) react with carbonyl compounds to form alcohols.
- Alkylating enolates: Enolates are reactive nucleophiles that can be alkylated with alkyl halides. Strong bases like LDA (lithium diisopropylamide) are often used to generate enolates.
- Williamson ether synthesis: Alkyl halides react with alkoxides to form ethers.
4. Acylation Reactions:
- Friedel-Crafts acylation: Acyl chlorides react with aromatic compounds in the presence of a Lewis acid catalyst (e.g., AlCl3) to form ketones.
- Esterification: Carboxylic acids react with alcohols in the presence of an acid catalyst to form esters.
Strategies for Efficient Reagent Selection
- Consult the literature: Extensive research on similar syntheses in academic journals and databases is crucial. This can provide valuable insights into successful reaction conditions and reagent choices.
- Consider protecting groups: If multiple functional groups are present that could interfere with each other, protecting groups might be necessary to selectively activate or deactivate certain functionalities. Common protecting groups include TBS (tert-butyldimethylsilyl) for alcohols and Boc (tert-butoxycarbonyl) for amines.
- Optimize reaction conditions: Careful optimization of reaction parameters, such as temperature, solvent, and concentration, can significantly improve yield and selectivity.
- Use spectroscopic techniques: Techniques like NMR and IR spectroscopy are essential for monitoring reaction progress and identifying products.
Advanced Considerations in Reagent Selection: Stereoselectivity and Regioselectivity
Achieving high levels of stereoselectivity and regioselectivity is often a critical challenge in organic synthesis. This often requires the careful selection of chiral reagents or catalysts. For instance:
- Chiral reducing agents: Reagents like Noyori's catalyst allow for the asymmetric reduction of ketones to yield chiral alcohols with high enantioselectivity.
- Sharpless epoxidation: This reaction uses a chiral titanium catalyst to selectively epoxidize allylic alcohols.
- Asymmetric alkylations: Chiral auxiliaries or catalysts can be employed to achieve high enantioselectivity in alkylation reactions.
Conclusion: A Holistic Approach to Successful Synthesis
Successfully completing an organic synthesis requires a multifaceted approach. This includes meticulous retrosynthetic analysis, careful consideration of reaction mechanisms, thorough literature research, and, most importantly, a deep understanding of reagent properties and reaction conditions. By employing these strategies, and consistently optimizing reaction conditions, chemists can build complex molecules efficiently and selectively. Remember that safety should always be a primary consideration throughout the synthesis, and proper handling and disposal of reagents are crucial. The process is iterative, and experimentation is often necessary to optimize yields and achieve the desired level of purity and stereoselectivity. Continuous learning and adapting to new synthetic methodologies and reagent developments are key to becoming a proficient organic chemist.
Latest Posts
Related Post
Thank you for visiting our website which covers about Complete The Synthesis Below By Selecting Or Drawing The Reagents. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
