Click On Each Chiral Center In The Cholesterol Derivative Below.
Holbox
Mar 28, 2025 · 6 min read
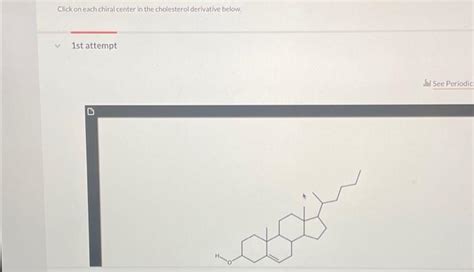
Table of Contents
- Click On Each Chiral Center In The Cholesterol Derivative Below.
- Table of Contents
- Click on Each Chiral Center in the Cholesterol Derivative Below
- Understanding Chiral Centers
- The Importance of Chirality in Biology
- Identifying Chiral Centers in a Cholesterol Derivative: A Step-by-Step Approach
- Visualizing the Chiral Centers
- Implications of Chiral Centers in Cholesterol Derivatives
- Pharmacological Relevance
- Metabolic Pathways
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Click on Each Chiral Center in the Cholesterol Derivative Below
Cholesterol, a ubiquitous steroid in animal tissues, plays a crucial role in cell membrane structure and function, hormone synthesis, and bile acid production. Its intricate structure, characterized by multiple chiral centers, is responsible for its diverse biological activities. Understanding the stereochemistry of cholesterol and its derivatives is paramount in comprehending their interactions with biological systems and designing effective therapeutic interventions. This article will delve into the identification of chiral centers within a cholesterol derivative, focusing on the principles of stereochemistry and their implications. While we cannot literally "click" on a molecular structure within this text, we will meticulously identify each chiral center and explain the reasoning behind our designation.
Understanding Chiral Centers
Before we embark on identifying the chiral centers in a specific cholesterol derivative, let's solidify our understanding of the fundamental concept. A chiral center, also known as a stereocenter or asymmetric carbon, is a carbon atom bonded to four different groups. This asymmetry leads to the existence of stereoisomers – molecules with the same connectivity but different spatial arrangements of atoms. These stereoisomers are non-superimposable mirror images of each other, a phenomenon known as chirality.
The Importance of Chirality in Biology
Chirality is profoundly important in biological systems. Enzymes, the biological catalysts responsible for countless metabolic processes, often exhibit high selectivity towards one stereoisomer over another. This means that a drug's effectiveness and even its toxicity can be drastically influenced by its chirality. For instance, one enantiomer of a drug might be highly therapeutic, while the other might be inactive or even harmful. This is why understanding and controlling the stereochemistry of pharmaceutical compounds is crucial in drug development and clinical application.
Identifying Chiral Centers in a Cholesterol Derivative: A Step-by-Step Approach
To effectively identify chiral centers, we need a structural representation of the cholesterol derivative. Let's assume we are working with a simplified cholesterol derivative for illustrative purposes. (Note: A visual representation would be ideal here, but since we cannot directly insert images, we will rely on a textual description. Imagine a cholesterol molecule with modifications to some of its functional groups.)
Let’s consider a hypothetical cholesterol derivative, modified at the 3- and 7-positions. We will focus on identifying chiral centers in the core steroid structure. The specific modifications at positions 3 and 7 won't necessarily alter the chirality of the original chiral centers, unless the modifications themselves introduce new chiral centers.
Step 1: Locate all carbon atoms. Carefully examine the structure, and identify all the carbon atoms in the steroid nucleus and side chains.
Step 2: Identify carbon atoms bonded to four different groups. For each carbon atom, examine the four groups attached to it. If all four groups are different, you have identified a chiral center. Remember that identical groups, even if they are positioned differently in space, do not constitute a chiral center.
Step 3: Systematic Examination of the Steroid Nucleus. Starting from the A-ring, meticulously assess each carbon atom. The carbons at positions 5, 8, 9, 10, and 13 are usually chiral centers in cholesterol and its derivatives. Let's analyze these positions individually in our hypothetical derivative:
-
Carbon 5: This carbon atom typically bears a methyl group, a hydrogen atom, and two carbons within the steroid ring system. This carbon atom almost always possesses four different groups. This is a chiral center.
-
Carbon 8: This carbon is usually bonded to methyl group, hydrogen, and two carbons within the steroid ring system. This is a chiral center.
-
Carbon 9: This carbon atom is generally connected to a methyl group, hydrogen, and two carbons within the steroid ring system. This is a chiral center.
-
Carbon 10: This carbon usually forms connections with a methyl group, a hydrogen, and two carbons forming part of the steroid ring system. This is a chiral center.
-
Carbon 13: This carbon often binds to a methyl group, hydrogen, and two carbons creating the steroid ring structure. This is a chiral center.
Step 4: Analysis of the Side Chain. The side chain of cholesterol typically contains additional chiral centers. The specific number and location will depend on the structure of the derivative. However, the general pattern is that any carbon atom in the side chain that has four different substituents will be a chiral center.
Step 5: Considering Modifications at Positions 3 and 7. The hypothetical modifications at positions 3 and 7 might introduce new chiral centers or alter existing ones. This requires carefully considering the nature of the modifications and their influence on the surrounding atoms. For example: If a hydroxyl group (-OH) was added at position 3 or 7, this would introduce a new asymmetric carbon. If the modification is a substitution for a hydrogen atom, the new group will need to be compared to the pre-existing substituent. If it is different from all other substituents, a chiral center is added.
Visualizing the Chiral Centers
It is crucial to reiterate that a visual representation would significantly aid in this process. Software packages such as ChemDraw or similar molecular modeling tools are invaluable for visualizing molecular structures and identifying chiral centers interactively. By rotating the molecule and examining each carbon atom individually, one can confidently determine whether the four substituents are unique.
Implications of Chiral Centers in Cholesterol Derivatives
The presence of multiple chiral centers in cholesterol and its derivatives leads to a vast number of possible stereoisomers. Each stereoisomer can exhibit different physical and chemical properties, influencing its biological activity.
Pharmacological Relevance
Many cholesterol-lowering drugs and other medications interact with cholesterol or its derivatives. Understanding the stereochemistry of these interactions is crucial for developing effective and safe therapies. The specific interaction between a drug and its target molecule is highly dependent on the three-dimensional arrangement of atoms, meaning chirality plays a significant role. One enantiomer might be highly effective, while its mirror image could be completely inactive or even toxic.
Metabolic Pathways
The metabolism of cholesterol and its derivatives involves enzymatic reactions that are highly stereospecific. This means that enzymes only interact with a specific stereoisomer of a cholesterol derivative. The stereochemical configuration of a cholesterol molecule will influence its path through the body’s metabolic process. This is significant for determining the bioavailability and effectiveness of pharmaceuticals and also the effects of dietary cholesterol.
Conclusion
Identifying chiral centers in cholesterol derivatives requires a systematic approach that combines knowledge of organic chemistry principles with careful analysis of the molecule's structure. The presence of multiple chiral centers in cholesterol and its derivatives has profound implications for their biological activity, metabolic pathways, and pharmacological applications. A thorough understanding of stereochemistry is essential for researchers and professionals working in related fields, including medicinal chemistry, biochemistry, and pharmacology. The use of molecular modeling software can significantly enhance this process, providing a visual and interactive approach to identifying and analyzing chiral centers within complex molecules.
Latest Posts
Latest Posts
-
Descent With Modification Describes The Process Of Multiple Choice Question
Mar 31, 2025
-
Being Surprised At Paying 20 A Plate
Mar 31, 2025
-
Standard Costs Are Used In The Calculation Of
Mar 31, 2025
-
When Trading With More Developed Countries
Mar 31, 2025
-
Special Education In Contemporary Society An Introduction To Exceptionality
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Click On Each Chiral Center In The Cholesterol Derivative Below. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
