Click On All The Electrophiles Then Check Your Answer
Holbox
Mar 29, 2025 · 5 min read
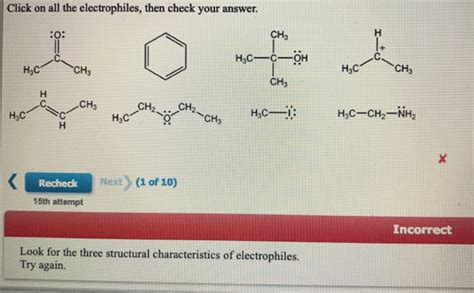
Table of Contents
- Click On All The Electrophiles Then Check Your Answer
- Table of Contents
- Click on All the Electrophiles: Then Check Your Answer – A Deep Dive into Electrophilic Chemistry
- Understanding Electrophiles: Electron-Loving Species
- Key Characteristics of Electrophiles:
- Identifying Electrophiles: A Practical Approach
- Common Examples of Electrophiles
- Carbonyl Compounds:
- Alkyl Halides:
- Acid Halides:
- Imines and Nitriles:
- Carbocations:
- Nucleophiles: The Counterparts to Electrophiles
- Electrophilic Addition Reactions: A Key Reaction Type
- Electrophilic Aromatic Substitution: Another Important Reaction Type
- The Importance of Electrophiles in Organic Synthesis
- Beyond the Click: Deeper Understanding through Practice
- Conclusion: Mastering Electrophilic Chemistry
- Latest Posts
- Latest Posts
- Related Post
Click on All the Electrophiles: Then Check Your Answer – A Deep Dive into Electrophilic Chemistry
Organic chemistry can feel like navigating a dense jungle, especially when you encounter concepts like electrophiles and nucleophiles. Understanding the difference between these two fundamental players is crucial for mastering organic reaction mechanisms. This comprehensive guide will not only help you identify electrophiles but also delve deep into their properties, reactions, and importance in organic synthesis. We'll tackle the question, "Click on all the electrophiles," by providing a robust understanding that goes beyond simple identification.
Understanding Electrophiles: Electron-Loving Species
At its core, an electrophile is an electron-deficient species. This means it possesses a positive charge, a partially positive charge (δ+), or an empty orbital capable of accepting electrons. Its very nature compels it to seek out electron-rich species to form a new chemical bond. Think of it as an electron "lover," hence the name "electrophile."
Key Characteristics of Electrophiles:
-
Positive Charge: Compounds with a full positive charge, like carbocations (e.g., CH₃⁺) are strong electrophiles. The positive charge attracts electrons intensely.
-
Partial Positive Charge (δ+): Molecules with polar bonds, where one atom is more electronegative than the other, will have a partially positive charge on the less electronegative atom. This partial positive charge makes it electrophilic. A classic example is the carbonyl carbon in aldehydes and ketones (C=O). The oxygen atom is more electronegative, pulling electron density away from the carbon, giving it a δ+ charge.
-
Empty Orbitals: Species with vacant orbitals, such as Lewis acids (like BF₃ or AlCl₃), can accept electron pairs. These empty orbitals are electron-deficient sites, making them strong electrophiles.
Identifying Electrophiles: A Practical Approach
Identifying electrophiles in a given molecule requires careful consideration of its structure and bonding. Here's a step-by-step approach:
-
Look for Positive Charges: The presence of a full positive charge immediately signifies a strong electrophile.
-
Identify Polar Bonds: Examine the molecule for polar bonds. The atom with the partial positive charge (δ+) will be the electrophilic center. Common examples include C=O, C-X (where X is a halogen), and C-N bonds.
-
Check for Empty Orbitals: Look for atoms with less than a full octet of electrons. These atoms actively seek electrons to complete their octet, making them electrophiles.
-
Consider Resonance Structures: Sometimes, the electrophilic character isn't immediately obvious. Drawing resonance structures can reveal partially positive charges that indicate electrophilic sites.
Common Examples of Electrophiles
Let's explore some common examples across different functional groups:
Carbonyl Compounds:
-
Aldehydes and Ketones: The carbonyl carbon (C=O) is electrophilic due to the oxygen's higher electronegativity. This makes them susceptible to nucleophilic attack.
-
Carboxylic Acids and Esters: Similar to aldehydes and ketones, the carbonyl carbon in carboxylic acids and esters is electrophilic.
Alkyl Halides:
- The carbon atom bonded to the halogen is electrophilic. The halogen's electronegativity creates a partial positive charge (δ+) on the carbon.
Acid Halides:
- The carbonyl carbon in acid halides is highly electrophilic, even more so than in aldehydes, ketones, or carboxylic acids due to the high electronegativity of the halogen.
Imines and Nitriles:
- The carbon atom in imines (C=N) and nitriles (C≡N) is electrophilic because of the nitrogen's electronegativity.
Carbocations:
- Carbocations are extremely strong electrophiles due to their full positive charge. They are highly reactive intermediates in many organic reactions.
Nucleophiles: The Counterparts to Electrophiles
To truly grasp the concept of electrophiles, it's essential to understand their counterparts: nucleophiles. Nucleophiles are electron-rich species that donate electrons to form a new bond with an electrophile. They have a lone pair of electrons or a π-bond available for donation. Common nucleophiles include hydroxide ions (OH⁻), amines (R₃N), and halides (Cl⁻, Br⁻, I⁻).
Electrophilic Addition Reactions: A Key Reaction Type
One of the most common reaction types involving electrophiles is electrophilic addition. This typically occurs with unsaturated compounds, such as alkenes and alkynes. The reaction involves two steps:
-
Electrophilic Attack: The electrophile attacks the electron-rich π-bond of the alkene or alkyne, forming a carbocation intermediate.
-
Nucleophilic Attack: A nucleophile then attacks the carbocation, forming a new σ-bond and completing the addition reaction.
Electrophilic Aromatic Substitution: Another Important Reaction Type
Another important reaction class involving electrophiles is electrophilic aromatic substitution. Here, an electrophile replaces a hydrogen atom on an aromatic ring. This reaction typically proceeds through a two-step mechanism:
-
Electrophilic Attack: The electrophile attacks the electron-rich aromatic ring, forming a resonance-stabilized carbocation intermediate (also called a arenium ion).
-
Proton Loss: A proton is then removed from the carbocation, regenerating the aromaticity of the ring and resulting in the substituted aromatic compound.
The Importance of Electrophiles in Organic Synthesis
Electrophiles are central to a vast array of organic reactions and are essential building blocks in many organic synthesis strategies. Their ability to react with nucleophiles allows for the construction of complex organic molecules from simpler starting materials. Understanding electrophilic reactivity is fundamental to designing and predicting the outcome of organic reactions.
Beyond the Click: Deeper Understanding through Practice
While a simple "click on all the electrophiles" exercise provides a basic understanding, true mastery comes through practice. Working through numerous examples, solving problems, and applying your knowledge to different scenarios is vital. Consider these further exercises to solidify your understanding:
- Draw the structures of various organic molecules and identify all electrophilic sites.
- Predict the products of electrophilic addition and substitution reactions.
- Design synthetic pathways to synthesize specific molecules utilizing electrophilic reactions.
- Explore the mechanisms of different reactions that involve electrophiles.
Conclusion: Mastering Electrophilic Chemistry
This detailed exploration of electrophiles goes beyond the simple task of clicking on them. It provides a comprehensive understanding of their nature, properties, and importance in organic chemistry. By mastering the concepts outlined above and diligently practicing, you'll develop a strong foundation in organic reaction mechanisms, enabling you to navigate the complexities of organic synthesis with confidence. Remember, consistent effort and a deep understanding of the underlying principles are key to success in organic chemistry. So, get practicing, and soon you'll be a pro at identifying and understanding these essential electron-loving species!
Latest Posts
Latest Posts
-
Is Balloon Vine Bigger Than Golden Rain Tree
Apr 01, 2025
-
Unused Live Ammunition Should Be Inventoried And Then
Apr 01, 2025
-
The Discount On Bonds Payable Account
Apr 01, 2025
-
Moles And Chemical Formulas Report Sheet Answers
Apr 01, 2025
-
The Narrower The Definition Of A Product
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Click On All The Electrophiles Then Check Your Answer . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
