Classify Each Molecule As An Aldehyde Ketone Or Neither
Holbox
Mar 13, 2025 · 5 min read
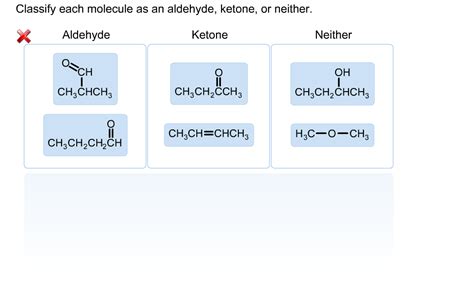
Table of Contents
Classify Each Molecule as an Aldehyde, Ketone, or Neither: A Comprehensive Guide
Identifying aldehydes and ketones is a fundamental skill in organic chemistry. These carbonyl-containing compounds, characterized by a carbon atom double-bonded to an oxygen atom (C=O), are ubiquitous in nature and crucial in various industrial applications. However, distinguishing between aldehydes and ketones requires a keen understanding of their structural differences. This comprehensive guide will equip you with the knowledge to confidently classify molecules as aldehydes, ketones, or neither. We'll explore the defining characteristics of each functional group, delve into examples, and troubleshoot common classification challenges.
Understanding Carbonyl Groups: The Heart of Aldehydes and Ketones
Before we dive into the classification process, let's establish a firm understanding of the carbonyl group (C=O). This functional group is the defining feature of both aldehydes and ketones. The carbonyl carbon is sp<sup>2</sup> hybridized, meaning it possesses three sigma bonds and one pi bond. The double bond between carbon and oxygen is polar, owing to the higher electronegativity of oxygen. This polarity significantly influences the reactivity of both aldehydes and ketones.
The crucial difference lies in the atoms bonded to the carbonyl carbon:
-
Aldehydes: The carbonyl carbon in an aldehyde is bonded to at least one hydrogen atom and one other carbon atom (or hydrogen atom). The general formula for an aldehyde is RCHO, where R represents an alkyl or aryl group (or hydrogen). The carbonyl group is always at the end of a carbon chain.
-
Ketones: The carbonyl carbon in a ketone is bonded to two carbon atoms (or one carbon and one alkyl/aryl group). The general formula for a ketone is RCOR', where R and R' represent alkyl or aryl groups. The carbonyl group is located within the carbon chain.
Classifying Molecules: A Step-by-Step Approach
Let's use a systematic approach to classify molecules. To determine if a molecule is an aldehyde, a ketone, or neither, follow these steps:
-
Identify the Carbonyl Group: Look for a C=O group. If it's absent, the molecule is neither an aldehyde nor a ketone.
-
Examine the Atoms Bonded to the Carbonyl Carbon: This is the crucial step.
-
If the carbonyl carbon is bonded to at least one hydrogen atom and at least one other carbon atom (or another hydrogen atom): It's an aldehyde.
-
If the carbonyl carbon is bonded to two carbon atoms (or one carbon and one alkyl/aryl group): It's a ketone.
-
If neither of the above conditions is met: It's neither an aldehyde nor a ketone.
-
Examples and Illustrations
Let's analyze some examples to solidify our understanding:
Example 1: Formaldehyde (HCHO)
Formaldehyde possesses a carbonyl group (C=O). The carbonyl carbon is bonded to two hydrogen atoms. Therefore, formaldehyde is an aldehyde.
Example 2: Acetone (CH<sub>3</sub>COCH<sub>3</sub>)
Acetone also contains a carbonyl group. However, the carbonyl carbon is bonded to two methyl groups (CH<sub>3</sub>). Acetone is a ketone.
Example 3: Ethanol (CH<sub>3</sub>CH<sub>2</sub>OH)
Ethanol contains an oxygen atom, but it's part of a hydroxyl group (-OH), not a carbonyl group. Ethanol is neither an aldehyde nor a ketone.
Example 4: Benzaldehyde (C<sub>6</sub>H<sub>5</sub>CHO)
Benzaldehyde features a carbonyl group where the carbonyl carbon is bonded to a hydrogen atom and a phenyl group (C<sub>6</sub>H<sub>5</sub>). Benzaldehyde is an aldehyde.
Example 5: Cyclohexanone (C<sub>6</sub>H<sub>10</sub>O)
Cyclohexanone possesses a carbonyl group within its cyclic structure. The carbonyl carbon is bonded to two carbon atoms within the ring. Cyclohexanone is a ketone.
Example 6: Acetic Acid (CH<sub>3</sub>COOH)
Acetic acid contains a carbonyl group, but it's part of a carboxyl group (-COOH). The presence of the hydroxyl group (-OH) bonded directly to the carbonyl carbon differentiates it from aldehydes and ketones. Acetic acid is neither an aldehyde nor a ketone.
Advanced Scenarios and Troubleshooting
While the basic classification is straightforward, some molecules can present more complex scenarios:
1. Cyclic Structures: In cyclic structures, carefully examine the atoms directly bonded to the carbonyl carbon to determine if it's an aldehyde or a ketone. Remember, an aldehyde will always have at least one hydrogen atom bonded to the carbonyl carbon.
2. Polyfunctional Molecules: Molecules containing multiple functional groups might possess both aldehyde and ketone groups, or a carbonyl group alongside other functional groups. In such cases, identify each functional group individually.
Applications of Aldehydes and Ketones
Understanding the classification of aldehydes and ketones is crucial due to their widespread applications:
-
Aldehydes: Used in the production of resins, plastics, and solvents. Formaldehyde, the simplest aldehyde, is a crucial building block in various industrial processes. Other aldehydes are used in fragrances and flavorings.
-
Ketones: Acetone, the simplest ketone, is an excellent solvent used extensively in industries like nail polish remover and paint thinner. Other ketones play vital roles in biological systems and are found in various natural products.
Conclusion: Mastering Aldehyde and Ketone Classification
The ability to accurately classify molecules as aldehydes, ketones, or neither is a foundational skill in organic chemistry. By systematically examining the structure of the molecule, focusing on the atoms bonded to the carbonyl carbon, you can confidently categorize any given molecule. Understanding these functional groups is not only essential for academic pursuits but also provides a solid foundation for comprehending the properties and applications of these crucial organic compounds across various fields, from industrial chemistry to biochemistry. Remember to practice regularly to develop a strong intuition and become proficient in identifying these important functional groups.
Latest Posts
Latest Posts
-
An Example Of An Institutional Coi Is
Mar 13, 2025
-
The Interest Rate That Banks Charge Their Best Customers
Mar 13, 2025
-
Which Nims Component Includes The Incident Command System Ics
Mar 13, 2025
-
Which Nims Structure Makes Cooperative Multi Agency Decisions
Mar 13, 2025
-
Linear Modeling Of Nyc Mta Transit Fares
Mar 13, 2025
Related Post
Thank you for visiting our website which covers about Classify Each Molecule As An Aldehyde Ketone Or Neither . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
