Choose The Best Lewis Structure For Ch2cl2
Holbox
Mar 14, 2025 · 5 min read
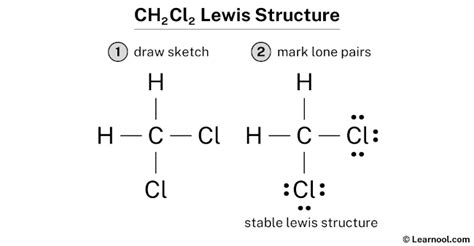
Table of Contents
Choosing the Best Lewis Structure for CH₂Cl₂: A Comprehensive Guide
Dichloromethane (CH₂Cl₂) is a simple yet illustrative molecule for understanding Lewis structures and the principles of formal charge minimization. While seemingly straightforward, selecting the best Lewis structure requires careful consideration of several factors, including octet rule adherence, formal charge distribution, and electronegativity differences. This comprehensive guide delves into the process of constructing and evaluating Lewis structures for CH₂Cl₂, ultimately determining the most accurate representation.
Understanding Lewis Structures
A Lewis structure, also known as a Lewis dot diagram, is a simplified representation of a molecule's valence electrons. It shows the arrangement of atoms and the bonding electrons (shared pairs) and lone pairs of electrons around each atom. These diagrams are crucial for predicting molecular geometry, polarity, and reactivity. The core principles governing Lewis structure construction include:
-
Valence Electrons: Determining the total number of valence electrons is the first step. Carbon has four, hydrogen has one, and chlorine has seven. For CH₂Cl₂, the total number of valence electrons is 4 + (2 × 1) + (2 × 7) = 20.
-
Octet Rule: Most atoms strive to achieve a stable electron configuration with eight electrons in their outermost shell (octet). Hydrogen, being a first-row element, is an exception, requiring only two electrons (duet).
-
Bonding and Lone Pairs: Electrons are depicted as dots or lines. A single line represents a single bond (two electrons), while a double line represents a double bond (four electrons). Lone pairs are pairs of electrons not involved in bonding.
Possible Lewis Structures for CH₂Cl₂
Several Lewis structures can be initially drawn for CH₂Cl₂, but most will be incorrect due to violation of the octet rule or unfavorable formal charges. Let's examine some possibilities and analyze their validity.
Structure 1: Incorrect Placement of Electrons
This structure might attempt to place all 20 electrons around the carbon atom, which violates the octet rule for chlorine atoms. This leads to an unstable and inaccurate representation.
Structure 2: Violating Octet Rule for Chlorine
This structure might incorrectly place only a single bond between carbon and chlorine and not satisfy the octet rule for both chlorine atoms. The chlorine atoms would have only 7 electrons, leading to an unstable structure.
Structure 3: The Correct Lewis Structure
This structure places all atoms in a tetrahedral arrangement, with single bonds between carbon and each hydrogen and chlorine atom. This structure accurately reflects the bonding in dichloromethane. Each atom (except hydrogen) achieves a stable octet, and this representation is the most accurate and stable.
Evaluating Lewis Structures: Formal Charges
Formal charge is a tool to assess the plausibility of a Lewis structure. It represents the difference between the number of valence electrons an atom has in its neutral state and the number of electrons assigned to it in the Lewis structure. The formula for calculating formal charge is:
Formal Charge = (Valence Electrons) - (Non-bonding Electrons) - ½(Bonding Electrons)
Analyzing Formal Charges for CH₂Cl₂ (Structure 3)
- Carbon: Formal Charge = 4 - 0 - ½(8) = 0
- Hydrogen (each): Formal Charge = 1 - 0 - ½(2) = 0
- Chlorine (each): Formal Charge = 7 - 6 - ½(2) = 0
The formal charges of all atoms in the correct Lewis structure are zero. This signifies a stable and preferred structure because structures with zero formal charges are generally more stable than those with non-zero formal charges.
Influence of Electronegativity
Electronegativity, the ability of an atom to attract electrons towards itself in a chemical bond, also plays a role in selecting the best Lewis structure. Chlorine is significantly more electronegative than carbon and hydrogen. In the correct Lewis structure, the electrons in the C-Cl bonds are slightly shifted towards chlorine. This partial negative charge on chlorine and partial positive charge on carbon further validates the chosen structure.
Molecular Geometry and VSEPR Theory
The correct Lewis structure (Structure 3) dictates the molecular geometry of CH₂Cl₂. According to VSEPR (Valence Shell Electron Pair Repulsion) theory, the four electron pairs around the central carbon atom (two bonding pairs with hydrogen, two bonding pairs with chlorine) will arrange themselves tetrahedrally to minimize repulsions. This tetrahedral geometry confirms the accuracy of our chosen Lewis structure.
Resonance Structures (Absence in CH₂Cl₂)
Resonance structures arise when multiple valid Lewis structures can be drawn for a molecule, and the actual structure is a hybrid of these contributing structures. In the case of CH₂Cl₂, there are no resonance structures. The correct Lewis structure (Structure 3) is the only plausible and stable representation.
Importance of Choosing the Best Lewis Structure
Selecting the most accurate Lewis structure is crucial because it forms the foundation for understanding and predicting several molecular properties:
-
Molecular Geometry: The arrangement of atoms influences the physical and chemical properties of the molecule.
-
Bond Polarity: The electronegativity differences between atoms determine bond polarity and contribute to the overall dipole moment of the molecule. Understanding the bond polarity in CH₂Cl₂ (polar due to the difference between C-H and C-Cl bonds) allows us to understand its properties, such as its solubility and boiling point.
-
Molecular Polarity: The overall polarity of a molecule depends on both the polarity of individual bonds and molecular geometry.
-
Reactivity: The electron distribution as depicted in the Lewis structure influences the molecule's reactivity towards other chemical species.
-
Spectroscopic Properties: Lewis structures help in understanding and interpreting spectroscopic data, such as infrared and NMR spectroscopy.
Conclusion
Determining the best Lewis structure for CH₂Cl₂ involves a systematic approach. We begin by calculating the total valence electrons and then construct a plausible structure adhering to the octet rule (or duet rule for hydrogen). Formal charge analysis plays a vital role in selecting the most stable structure, with structures having zero formal charges generally preferred. Considering electronegativity differences and understanding molecular geometry based on VSEPR theory further corroborate the accuracy of the chosen Lewis structure. The best Lewis structure for CH₂Cl₂ is the tetrahedral arrangement with single bonds between carbon and hydrogen and carbon and chlorine, resulting in zero formal charges and a stable octet for all atoms except hydrogen (which has a stable duet). This structure is not only the simplest but also the most accurate representation of the bonding in dichloromethane. This detailed analysis highlights the importance of understanding and applying these principles to correctly represent molecular structures, a cornerstone of fundamental chemistry. This correct representation allows for accurate prediction of molecular properties and facilitates further exploration of its chemical behavior.
Latest Posts
Latest Posts
-
The Opportunity Cost Of An Action Is Always Equal To
Mar 15, 2025
-
Providing And Recording Documents Are Performed By
Mar 15, 2025
-
The Most Significant Hazard Associated With Splinting Is
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Choose The Best Lewis Structure For Ch2cl2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
