Calculate The Heat Of Reaction In Trial 1
Holbox
Mar 17, 2025 · 7 min read
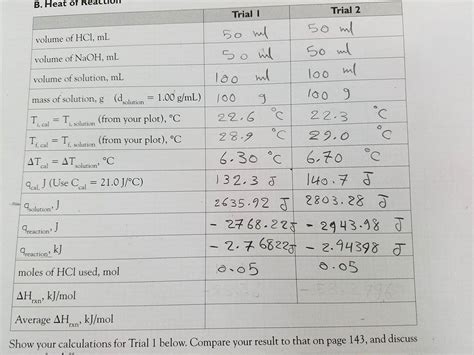
Table of Contents
Calculating the Heat of Reaction in Trial 1: A Comprehensive Guide
Determining the heat of reaction, also known as the enthalpy change (ΔH), for a specific trial is crucial in understanding the thermodynamics of a chemical reaction. This process involves careful measurements and calculations, and understanding the underlying principles is key to accurate results. This article provides a detailed, step-by-step guide on calculating the heat of reaction for Trial 1, focusing on common methodologies and potential sources of error. We will cover various scenarios, from simple calorimetry experiments to more complex reactions.
Understanding the Fundamentals: Heat Transfer and Calorimetry
Before diving into the calculations, let's review the fundamental principles governing heat transfer and its measurement using calorimetry.
Heat Transfer: The First Law of Thermodynamics
At the heart of this process lies the First Law of Thermodynamics, which states that energy cannot be created or destroyed, only transferred or changed from one form to another. In a chemical reaction, the heat released or absorbed is directly related to the change in enthalpy (ΔH). Exothermic reactions release heat into the surroundings (ΔH < 0), while endothermic reactions absorb heat from the surroundings (ΔH > 0).
Calorimetry: Measuring Heat Transfer
Calorimetry is the experimental technique used to measure the heat transfer associated with a chemical or physical process. The most common type is constant-pressure calorimetry, which is often performed using a simple calorimeter, typically a coffee-cup calorimeter. This setup allows the reaction to occur at constant atmospheric pressure.
Calculating the Heat of Reaction for Trial 1: A Step-by-Step Approach
The specific steps involved in calculating the heat of reaction for Trial 1 depend on the type of calorimeter used and the specific experimental conditions. However, the general approach involves the following:
1. Data Collection: Gathering Essential Information
Before initiating any calculation, meticulously record all relevant data from Trial 1. This includes:
- Mass of reactants: Precisely measure the mass of each reactant used in Trial 1. Record this in grams (g). Any inaccuracies here will directly impact the final calculation.
- Initial temperature (Tᵢ): Record the temperature of the reactants before the reaction begins. Accuracy is crucial here. Use a thermometer with sufficient precision.
- Final temperature (Tƒ): Note the temperature of the reaction mixture after the reaction has reached completion and thermal equilibrium is established. Again, precision is paramount.
- Specific heat capacity of the solution (Cₛ): This value represents the amount of heat required to raise the temperature of 1 gram of the solution by 1 degree Celsius (or 1 Kelvin). The specific heat capacity of water is often used as an approximation (4.18 J/g°C), but this may vary depending on the solution's composition. For more accurate results, determine the specific heat capacity of the specific solution used in your experiment.
- Mass of the solution: This is the total mass of the reactants and any solvent used.
- Heat capacity of the calorimeter (C<sub>cal</sub>): This accounts for the heat absorbed by the calorimeter itself. If a simple coffee-cup calorimeter is used, this value is often negligible or can be determined through calibration experiments.
2. Calculating the Heat Absorbed by the Solution (q<sub>sol</sub>)
The heat absorbed by the solution (q<sub>sol</sub>) can be calculated using the following formula:
q<sub>sol</sub> = m<sub>sol</sub> × C<sub>s</sub> × ΔT
Where:
- m<sub>sol</sub> is the mass of the solution (g)
- C<sub>s</sub> is the specific heat capacity of the solution (J/g°C)
- ΔT is the change in temperature (Tƒ - Tᵢ) (°C)
Example: If the mass of the solution is 100 g, the specific heat capacity is 4.18 J/g°C, and the temperature change is 5°C, then:
q<sub>sol</sub> = 100 g × 4.18 J/g°C × 5°C = 2090 J
3. Calculating the Heat Absorbed by the Calorimeter (q<sub>cal</sub>)
If the heat capacity of the calorimeter (C<sub>cal</sub>) is significant, it must be included in the calculation:
q<sub>cal</sub> = C<sub>cal</sub> × ΔT
Where:
- C<sub>cal</sub> is the heat capacity of the calorimeter (J/°C)
- ΔT is the change in temperature (°C)
This value is added to q<sub>sol</sub> to get the total heat absorbed.
4. Calculating the Total Heat Transferred (q<sub>total</sub>)
The total heat transferred (q<sub>total</sub>) is the sum of the heat absorbed by the solution and the heat absorbed by the calorimeter:
q<sub>total</sub> = q<sub>sol</sub> + q<sub>cal</sub>
5. Calculating the Heat of Reaction (ΔH)
Finally, the heat of reaction (ΔH), expressed in Joules per mole (J/mol), is calculated by dividing the total heat transferred (q<sub>total</sub>) by the number of moles of the limiting reactant:
ΔH = q<sub>total</sub> / n
Where:
- q<sub>total</sub> is the total heat transferred (J)
- n is the number of moles of the limiting reactant (mol)
The number of moles is calculated using the molar mass of the limiting reactant and its mass used in the experiment.
Advanced Considerations and Potential Sources of Error
While the above steps provide a general framework, several factors can influence the accuracy of the results. Let's explore some of these:
Heat Loss to the Surroundings
A significant source of error in simple calorimetry experiments is heat loss to the surroundings. The calorimeter is not perfectly insulated, leading to some heat exchange with the environment. This results in an underestimation of the actual heat of reaction. Minimizing heat loss is crucial; consider using better insulation or performing the experiment in a controlled environment.
Incomplete Reactions
If the reaction in Trial 1 is not complete, the calculated heat of reaction will be lower than the actual value. Ensuring the reaction goes to completion is essential. This often requires sufficient reaction time and optimal conditions (temperature, concentration, etc.).
Specific Heat Capacity Variations
Using an approximate value for the specific heat capacity of the solution (e.g., assuming it's the same as water) introduces errors. The more accurately this value is determined, the better the results. Using a more sophisticated calorimeter might also help in accounting for specific heat capacity variations.
Calibration of the Calorimeter
For more accurate results, particularly with complex calorimeters, calibration is essential to determine the heat capacity of the calorimeter itself (C<sub>cal</sub>). This involves a separate experiment where a known amount of heat is introduced to the calorimeter, allowing the determination of C<sub>cal</sub>.
Different Reaction Types and Calorimetry Techniques
The methods described above apply primarily to simple reactions in a constant-pressure calorimeter. However, different reaction types and more sophisticated calorimetry techniques might be necessary for certain experiments.
Bomb Calorimetry (Constant-Volume Calorimetry)
For reactions involving gases or significant volume changes, bomb calorimetry is used. This technique maintains a constant volume, and the heat of reaction is calculated using a different formula that accounts for the pressure-volume work done.
More Complex Reactions
Reactions involving multiple steps or equilibrium processes necessitate more sophisticated approaches, including:
- Titration calorimetry: used for reactions that proceed slowly or involve complex equilibrium shifts.
- Differential scanning calorimetry (DSC): This technique measures the heat flow associated with transitions, such as melting points or glass transitions.
Conclusion: Ensuring Accuracy and Reliability
Accurate determination of the heat of reaction for Trial 1 (or any trial) requires careful experimental technique and precise calculations. Pay close attention to detail during data collection and consider potential sources of error. Understanding the principles of heat transfer and choosing the appropriate calorimetry technique are crucial for obtaining reliable results. By following these guidelines and using appropriate error analysis, you can ensure the accuracy and reliability of your calculated heat of reaction values. Remember that repeated trials are essential to confirm results and minimize the impact of random errors. Analyzing the data from multiple trials and calculating the average will often provide a more accurate reflection of the true heat of reaction.
Latest Posts
Latest Posts
-
Which Function Is Shown In The Graph Below
Mar 18, 2025
-
Endocytosis Moves Materials A Cell Via
Mar 18, 2025
-
Behaviorism Focuses On Making Psychology An Objective Science By
Mar 18, 2025
-
How To Link Chegg To Tinder
Mar 18, 2025
-
The Nucleus Of An Atom Contains
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Calculate The Heat Of Reaction In Trial 1 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
