C3h6o I2 Goes To C3h5io I- H
Holbox
Mar 19, 2025 · 5 min read
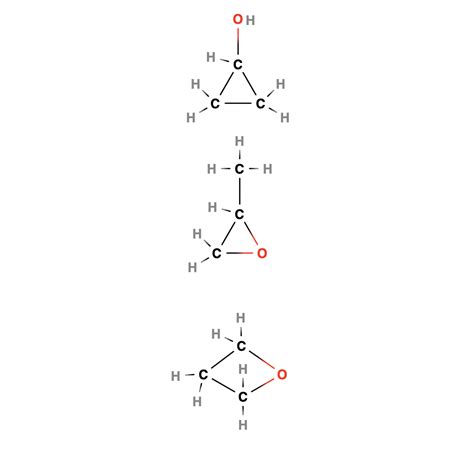
Table of Contents
Unveiling the Reaction Mechanism: C3H6O + I2 → C3H5IO + HI
The transformation of a carbonyl compound, represented by the general formula C₃H₆O, in the presence of iodine (I₂) to yield an iodo-substituted product (C₃H₅IO) and hydrogen iodide (HI) is a fascinating chemical reaction with implications across various fields. This article delves into the intricacies of this reaction mechanism, exploring the underlying principles, influencing factors, and potential applications. While the specific carbonyl compound isn't explicitly stated, the reaction's overall pathway can be analyzed and understood through a generalized approach.
Understanding the Reactants
Before diving into the mechanism, it's crucial to understand the properties of the reactants:
C₃H₆O (Unsaturated Carbonyl Compound)
The formula C₃H₆O represents a family of unsaturated carbonyl compounds, which can be ketones or aldehydes. Examples include:
- Acetone (CH₃COCH₃): A common solvent and ketone.
- Propanal (CH₃CH₂CHO): An aldehyde with a characteristic pungent odor.
- Allyl alcohol (CH₂=CHCH₂OH): An unsaturated alcohol, though not strictly a ketone or aldehyde, still possessing a reactive carbon-oxygen double bond.
The specific reactivity will differ depending on the structure of the C₃H₆O compound.
I₂ (Iodine)
Iodine, a diatomic molecule, is a relatively weak oxidizing agent compared to other halogens. Its ability to participate in this reaction hinges on its ability to undergo heterolytic cleavage, forming an electrophilic iodine cation (I⁺) and an iodide anion (I⁻). This will become crucial in the reaction's mechanistic steps.
Proposed Reaction Mechanism: A Step-by-Step Analysis
The reaction between C₃H₆O and I₂ likely proceeds through a multi-step mechanism involving nucleophilic attack and subsequent elimination. The exact steps might vary depending on the specific C₃H₆O molecule, but a general mechanism can be proposed:
Step 1: Electrophilic Attack by I₂
The iodine molecule (I₂) doesn't directly attack the carbonyl carbon. Instead, it likely undergoes heterolytic cleavage, possibly aided by a solvent or catalyst (not explicitly stated in the original reaction). This generates an electrophilic iodine cation (I⁺) and a nucleophilic iodide anion (I⁻). The I⁺ attacks the carbonyl oxygen, forming a positively charged intermediate:
C₃H₆O + I₂ ⇌ C₃H₆O⁺I + I⁻
This step is likely slow and rate-determining. The electrophilicity of I⁺ is enhanced by the electron-withdrawing nature of the oxygen atom in the carbonyl group.
Step 2: Nucleophilic Attack by I⁻
The iodide anion (I⁻), generated in step 1, acts as a nucleophile, attacking the carbon atom of the carbonyl group. This attack leads to the formation of a tetrahedral intermediate:
C₃H₆O⁺I + I⁻ → [C₃H₆(OI)I]
This intermediate is unstable due to the presence of four bonds to the carbon atom.
Step 3: Proton Transfer & Elimination
The tetrahedral intermediate undergoes a proton transfer and elimination reaction. A proton (H⁺) is abstracted from a neighboring carbon atom (alpha-carbon), leading to the formation of a double bond and the departure of a HI molecule. This results in the formation of the iodo-substituted product (C₃H₅IO):
[C₃H₆(OI)I] → C₃H₅IO + HI
The exact location of the iodine substitution on the C₃H₅IO molecule will depend on the structure of the starting carbonyl compound and the stability of the resulting intermediate. The most stable product will be favored.
Factors Influencing the Reaction
Several factors can influence the rate and yield of this reaction:
-
Nature of the carbonyl compound: The reactivity of the carbonyl compound significantly impacts the reaction rate. Ketones generally react slower than aldehydes due to steric hindrance. The presence of electron-donating or electron-withdrawing groups on the carbonyl compound also influences the reaction rate.
-
Solvent: The choice of solvent plays a crucial role. Polar solvents can stabilize the charged intermediates formed during the reaction, thus accelerating the reaction rate. Aprotic solvents are often preferred to avoid unwanted side reactions.
-
Temperature: Increasing the temperature generally increases the reaction rate, but excessive heat can lead to side reactions or decomposition of the products. An optimized temperature needs to be determined experimentally.
-
Presence of Catalysts: Certain catalysts might enhance the reaction rate by promoting the heterolytic cleavage of I₂ or stabilizing the intermediates. However, the specific catalyst (if any) would need to be identified and optimized for this reaction.
-
Concentration of Reactants: The concentration of both the carbonyl compound and iodine influence the reaction rate. Higher concentrations generally lead to faster reactions but might also lead to increased side products.
Potential Applications
The iodo-substituted products (C₃H₅IO) generated through this reaction have potential applications in:
-
Organic Synthesis: These compounds can serve as valuable intermediates in the synthesis of various organic molecules, especially those containing iodine. Iodine can be further manipulated or replaced with other functional groups.
-
Pharmaceutical Industry: Iodo-substituted compounds are found in several pharmaceuticals due to the unique properties of iodine. These products might show activity against various diseases.
-
Material Science: Iodo-compounds can be incorporated into polymeric materials, influencing their properties like conductivity or reactivity.
-
Analytical Chemistry: Iodinated compounds can be useful as reagents or markers in analytical techniques.
Further Research and Considerations
To gain a more comprehensive understanding of this reaction, several aspects warrant further investigation:
-
Kinetic Studies: Detailed kinetic studies are needed to determine the rate law and activation energy of the reaction, providing insights into the rate-determining step.
-
Computational Studies: Computational chemistry methods can provide valuable information about the reaction mechanism, energy profiles, and stability of the intermediates involved.
-
Substrate Scope: A broader study is needed on different C₃H₆O molecules to understand how their structure affects reactivity and product distribution.
-
Optimization of Reaction Conditions: Systematic optimization of reaction conditions (solvent, temperature, catalyst) is crucial for improving yield and selectivity.
Conclusion
The reaction of C₃H₆O with I₂ to produce C₃H₅IO and HI is a complex process involving a multi-step mechanism. Understanding the underlying principles, influencing factors, and potential applications of this reaction is vital for advancements in organic synthesis, material science, and other relevant fields. Further research is required to fully elucidate the reaction mechanism and optimize reaction conditions for various C₃H₆O substrates. This detailed analysis provides a solid foundation for future investigations and development in this area. The precise details of the reaction, including reaction kinetics, specific catalyst use (if any), and product distribution, require further experimentation and detailed analysis tailored to the specific C₃H₆O compound under consideration.
Latest Posts
Latest Posts
-
The Amount Of Inspection Needed Depends On And
Mar 20, 2025
-
Rn Community Health Online Practice 2023 B
Mar 20, 2025
-
Classify The Given Items With The Appropriate Group
Mar 20, 2025
-
Most Of The Western Progressive Reformers
Mar 20, 2025
-
In A Recent Poll Of 1500 Randomly Selected Eligible Voters
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about C3h6o I2 Goes To C3h5io I- H . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
