Bromobenzene Is Converted To A Compound With The Molecular Formula
Holbox
Mar 28, 2025 · 6 min read
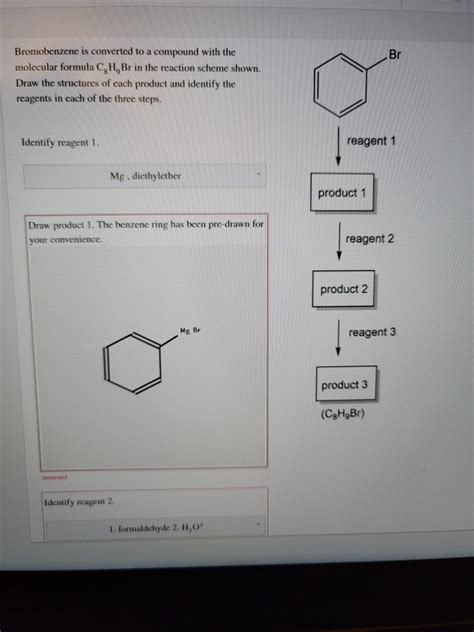
Table of Contents
- Bromobenzene Is Converted To A Compound With The Molecular Formula
- Table of Contents
- Bromobenzene Conversion: A Deep Dive into Reaction Pathways and Product Identification
- Understanding Bromobenzene's Reactivity
- Common Reactions of Bromobenzene: A Comprehensive Overview
- 1. Grignard Reagent Formation
- 2. Electrophilic Aromatic Substitution (EAS)
- 3. Nucleophilic Aromatic Substitution (SNAr)
- 4. Reduction Reactions
- 5. Metal-Catalyzed Coupling Reactions
- Factors Influencing Reaction Yield and Selectivity
- Conclusion: A Versatile Building Block
- Latest Posts
- Latest Posts
- Related Post
Bromobenzene Conversion: A Deep Dive into Reaction Pathways and Product Identification
Bromobenzene, a simple yet versatile aromatic compound, serves as a crucial building block in organic synthesis. Its unique reactivity profile allows for a wide array of transformations, leading to a diverse range of products. Understanding these transformations is crucial for anyone working in organic chemistry, from students to seasoned researchers. This article will explore the various pathways through which bromobenzene can be converted to different compounds, focusing on the identification of products based on their molecular formulas. We will delve into the mechanisms involved, considering factors influencing reaction yield and selectivity. The scope of this article encompasses a broad range of reactions, offering a comprehensive guide to understanding bromobenzene's synthetic utility.
Understanding Bromobenzene's Reactivity
Before delving into specific conversions, let's briefly examine the key aspects of bromobenzene's reactivity. The presence of the bromine atom significantly influences the reactivity of the benzene ring. Bromine is a deactivating and ortho/para-directing substituent. This means it reduces the overall reactivity of the benzene ring towards electrophilic aromatic substitution compared to benzene itself. However, the bromine atom still allows for reactions to proceed, albeit at a slower rate, predominantly at the ortho and para positions.
This directing effect is crucial in determining the product obtained in various reactions. Understanding the electronic effects of the bromine substituent is key to predicting the outcome of any transformation.
Common Reactions of Bromobenzene: A Comprehensive Overview
Several key reactions can transform bromobenzene into various compounds with different molecular formulas. Let's explore some of the most frequently used methods:
1. Grignard Reagent Formation
The conversion of bromobenzene to a Grignard reagent is a cornerstone reaction in organic chemistry. Treatment of bromobenzene with magnesium metal in anhydrous diethyl ether results in the formation of phenylmagnesium bromide (PhMgBr), a powerful nucleophile.
Reaction:
C₆H₅Br + Mg → C₆H₅MgBr
Molecular Formula of Product: C₆H₅MgBr
This Grignard reagent can then be utilized in a wide range of reactions, including:
- Carbonylation: Reacting with carbon dioxide followed by acidic workup yields benzoic acid (C₇H₆O₂).
- Addition to Carbonyl Compounds: Reacts with aldehydes and ketones to form secondary and tertiary alcohols respectively. The exact molecular formula of the product will depend on the specific carbonyl compound used.
- Reaction with Epoxides: Opening of epoxides to yield alcohols. Again, the molecular formula will vary depending on the epoxide used.
The versatility of the Grignard reagent makes it an invaluable tool for synthesizing a multitude of compounds from bromobenzene.
2. Electrophilic Aromatic Substitution (EAS)
Despite the deactivating nature of bromine, electrophilic aromatic substitution reactions can still be achieved, albeit under more vigorous conditions. Common EAS reactions include:
- Nitration: Treatment with concentrated nitric and sulfuric acids introduces a nitro group (-NO₂) onto the benzene ring. The major product is para-nitrobromobenzene.
Reaction:
C₆H₅Br + HNO₃/H₂SO₄ → C₆H₄BrNO₂ + H₂O
Molecular Formula of Product: C₆H₄BrNO₂
- Sulfonation: Reaction with fuming sulfuric acid introduces a sulfonic acid group (-SO₃H) onto the benzene ring. This reaction also favors para-substitution.
Reaction:
C₆H₅Br + H₂SO₄ (fuming) → C₆H₄BrSO₃H + H₂O
Molecular Formula of Product: C₆H₄BrSO₃H
- Halogenation: While bromobenzene already contains a bromine atom, further halogenation can occur, particularly with chlorine or iodine, to yield di- or tri-halogenated products. The precise position of the additional halogen(s) depends on the reaction conditions and the specific halogen used. The molecular formula will reflect the number and type of halogen atoms introduced.
3. Nucleophilic Aromatic Substitution (SNAr)
Under certain conditions, bromobenzene can undergo nucleophilic aromatic substitution. This typically requires the presence of strong electron-withdrawing groups on the benzene ring to activate it towards nucleophilic attack. For example, if we have a bromobenzene derivative with a strong electron-withdrawing group in the ortho or para position, a nucleophilic aromatic substitution can occur.
Example (with a strong electron-withdrawing group present): Let's assume we have a bromobenzene derivative with a nitro group in the para position (p-bromonitrobenzene). This compound can undergo nucleophilic aromatic substitution with a nucleophile like methoxide ion (CH₃O⁻).
Reaction:
C₆H₄BrNO₂ + CH₃O⁻ → C₆H₄OCH₃NO₂ + Br⁻
Molecular Formula of Product: C₇H₇NO₄
4. Reduction Reactions
The bromine atom in bromobenzene can be reduced to a hydrogen atom using various reducing agents. Common methods include:
- Catalytic Hydrogenation: Using hydrogen gas in the presence of a metal catalyst (like palladium on carbon) reduces the bromine atom to hydrogen.
Reaction:
C₆H₅Br + H₂ (Pd/C) → C₆H₆ + HBr
Molecular Formula of Product: C₆H₆ (Benzene)
- Lithium Aluminum Hydride (LiAlH₄): A powerful reducing agent, LiAlH₄ can also reduce the C-Br bond, although it's typically less preferred due to the possibility of side reactions.
5. Metal-Catalyzed Coupling Reactions
Bromobenzene participates in a variety of metal-catalyzed coupling reactions, which are powerful tools for forming carbon-carbon bonds.
-
Suzuki Coupling: Bromobenzene can react with boronic acids in the presence of a palladium catalyst to form biaryl compounds. The molecular formula of the product depends on the boronic acid used.
-
Stille Coupling: Similar to Suzuki coupling, Stille coupling uses organotin reagents and palladium catalysts to form carbon-carbon bonds. The product's molecular formula is determined by the organotin reagent employed.
-
Heck Reaction: This reaction involves the coupling of bromobenzene with alkenes in the presence of a palladium catalyst. The product is a substituted alkene, and the molecular formula depends on the alkene used.
-
Sonogashira Coupling: This reaction couples bromobenzene with terminal alkynes to yield substituted alkynes. Again, the molecular formula of the product depends on the alkyne used.
Factors Influencing Reaction Yield and Selectivity
Several factors significantly influence the yield and selectivity of these reactions:
- Reaction conditions: Temperature, solvent, concentration of reagents, and reaction time all play a crucial role.
- Presence of catalysts: Catalysts can significantly increase the reaction rate and improve selectivity.
- Nature of the substituents: The presence of other substituents on the benzene ring can affect both reactivity and regioselectivity.
- Steric hindrance: Bulky substituents can hinder the approach of reagents, leading to lower yields and altered selectivity.
Conclusion: A Versatile Building Block
Bromobenzene's reactivity profile allows for a diverse array of transformations. Understanding the reaction mechanisms and influencing factors is essential for predicting product outcomes and optimizing reaction conditions. This comprehensive overview highlights the versatility of bromobenzene as a key building block in organic synthesis, enabling the creation of a vast range of compounds with varying molecular formulas. Further research into specific reaction pathways and the application of advanced techniques can further enhance the understanding and utilization of bromobenzene in various chemical synthesis endeavors. The field of organic chemistry continues to evolve, constantly uncovering new and exciting applications for this fundamental aromatic compound. This article serves as a starting point for exploring the fascinating world of bromobenzene transformations and its significant contributions to the realm of organic synthesis.
Latest Posts
Latest Posts
-
Procedure 4 Testing The Extrinsic Eye Muscles
Apr 01, 2025
-
Give The Chemical Formulas For Each Of These Acids
Apr 01, 2025
-
Mrs Pierce Would Like To Enroll
Apr 01, 2025
-
To The Economist Total Cost Includes
Apr 01, 2025
-
Athletes Who Consume Adequate Carbohydrates Experience
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Bromobenzene Is Converted To A Compound With The Molecular Formula . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
