Below Is The Structure For The Antibiotic Mycomycin
Holbox
Apr 03, 2025 · 6 min read
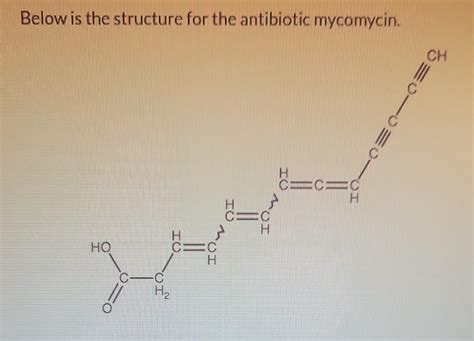
Table of Contents
- Below Is The Structure For The Antibiotic Mycomycin
- Table of Contents
- Mycomycin: Structure, Biosynthesis, and Biological Activity
- Understanding the Structure of Mycomycin
- Biosynthesis of Mycomycin: A Complex Metabolic Pathway
- Mycomycin's Biological Activity: Mechanism of Action and Applications
- Challenges and Future Directions in Mycomycin Research
- Conclusion: A Promising Antibiotic with Potential
- Latest Posts
- Latest Posts
- Related Post
Mycomycin: Structure, Biosynthesis, and Biological Activity
Mycomycin, a fascinating naturally occurring antibiotic, holds a unique position in the world of bioactive molecules. Its complex structure and potent biological activity have captivated researchers for decades, sparking extensive investigations into its biosynthesis and potential applications. This comprehensive article delves into the intricacies of mycomycin, exploring its structure, biosynthesis, mechanism of action, and promising applications.
Understanding the Structure of Mycomycin
Mycomycin, formally known as 10-[(Z)-1-hydroxyethenyl]-3,5,7-trimethyl-2,4,6,8-nonatetraenedioic acid, possesses a captivatingly complex structure. At its core lies a conjugated nonaene system – a chain of nine carbon atoms linked by alternating single and double bonds. This extended conjugated system is responsible for many of its unique properties, including its strong UV absorbance and characteristic yellow color.
Key Structural Features:
-
Conjugated Nonaene Chain: The backbone of the molecule is characterized by this extensive chain of conjugated double bonds, conferring significant structural rigidity and electronic delocalization. This feature is crucial for its interactions with biological targets.
-
Methyl Substitutions: Three methyl groups are strategically located along the polyene chain, influencing its overall shape and impacting its interactions with its biological environment. These methyl groups provide steric bulk and affect the conformation of the molecule.
-
Hydroxyethenyl Group: A critical functional group, the (Z)-1-hydroxyethenyl moiety located at the end of the conjugated system, plays a pivotal role in mycomycin's biological activity. Its hydroxyl group contributes to its polar character, affecting solubility and interaction with biological targets.
-
Dioic Acid Functionality: The presence of two carboxylic acid groups at either end of the molecule further enhances its polar nature and contributes to its overall reactivity and interaction with biological systems. These groups can participate in hydrogen bonding and other non-covalent interactions.
Structural Isomers and Conformational Considerations:
The conjugated nature of the nonaene chain introduces the possibility of cis-trans isomerism (geometric isomerism). Mycomycin's natural configuration, depicted in its formal name, is crucial for its biological activity. Slight alterations in the arrangement of double bonds can significantly diminish or even abolish its biological activity. The molecule also exhibits conformational flexibility, though the extended conjugated system restricts the degree of freedom. Understanding the preferred conformations is essential for comprehending its interactions with biological targets.
Biosynthesis of Mycomycin: A Complex Metabolic Pathway
The biosynthesis of mycomycin is a remarkable example of nature's sophisticated metabolic engineering. The exact pathway remains under investigation, but current research indicates a complex interplay of enzymatic reactions, likely involving polyketide synthase (PKS) systems.
Proposed Biosynthetic Steps:
-
Polyketide Chain Assembly: The foundational step involves the iterative condensation of acetate units (or malonyl-CoA derivatives) by a PKS enzyme complex. This assembly generates a linear polyketide chain, the precursor to the nonaene backbone. The enzyme meticulously controls the stereochemistry at each carbon, leading to the specific configuration found in mycomycin.
-
Chain Modification and Methylation: After the basic polyketide chain is formed, a series of modifications occur. These involve the introduction of methyl groups at specific positions through the action of methyltransferases. The precise timing and location of these methylation events are critical for the formation of the correct isomer.
-
Double Bond Introduction and Isomerization: The biosynthesis further entails the introduction of double bonds to create the conjugated nonaene system. Specific enzymes likely catalyze the creation of the double bonds and ensure the correct cis-trans isomerization of the double bonds, culminating in the desired Z-configuration of the molecule.
-
Hydroxyethenyl Group Formation: The addition of the (Z)-1-hydroxyethenyl group at the terminal end of the molecule is a crucial late-stage modification. This step likely involves the action of specific enzymes capable of incorporating this functional group into the growing polyketide chain.
-
Dioic Acid Formation: The two carboxylic acid groups are likely introduced through oxidation reactions of terminal methyl groups or other precursor functional groups. This process completes the synthesis of the mycomycin molecule.
Enzymatic Machinery and Genetic Control:
The biosynthetic pathway of mycomycin is governed by a complex network of enzymes and regulated by the genes encoding these enzymes. These genes are likely clustered together in the producing organism's genome, forming a biosynthetic gene cluster. Identifying and characterizing these genes provides crucial insights into the intricate steps of mycomycin biosynthesis.
Mycomycin's Biological Activity: Mechanism of Action and Applications
Mycomycin demonstrates potent antimicrobial activity, particularly against Gram-positive bacteria. Its mechanism of action is believed to be multifaceted, targeting multiple aspects of bacterial physiology.
Mechanism of Action:
-
Membrane Disruption: Mycomycin's amphipathic nature, with both polar and nonpolar regions, suggests its interaction with bacterial cell membranes. The conjugated nonaene system may embed within the membrane, altering its fluidity and integrity, leading to membrane disruption and cell death.
-
DNA Interaction: Studies suggest that mycomycin may also interact with bacterial DNA, potentially inhibiting DNA replication or transcription. The molecule's extended conjugated system could participate in intercalation or other interactions with DNA, causing damage to the genetic material.
-
Inhibition of Metabolic Pathways: It's plausible that mycomycin interferes with crucial metabolic pathways within the bacterial cell. Further research is needed to elucidate the specific metabolic targets affected by mycomycin.
Applications and Potential Uses:
-
Antibacterial Agent: The primary potential application of mycomycin is as an antibacterial agent, especially against drug-resistant bacterial strains. Its unique structure and mechanism of action could offer a novel approach in combating antibiotic resistance.
-
Anticancer Agent: Some studies suggest a potential anticancer activity of mycomycin. Its interaction with DNA and cell membranes could contribute to its cytotoxic effects on cancer cells. Further investigation is crucial to explore this potential therapeutic use.
-
Other Biological Activities: Mycomycin has also been shown to exhibit other biological activities, including antifungal and antiparasitic effects, though these are less well-studied compared to its antibacterial activity.
Challenges and Future Directions in Mycomycin Research
Despite its promising biological activity, several challenges hinder the widespread application of mycomycin.
-
Low Natural Abundance: Mycomycin is produced in relatively low amounts by its natural source, making its large-scale isolation and purification challenging and expensive.
-
Chemical Instability: Mycomycin is chemically unstable, prone to degradation under certain conditions, which poses challenges for its formulation and storage.
-
Toxicity: While showing promising antimicrobial activity, mycomycin's toxicity profile needs comprehensive investigation to ensure its safe use in therapeutic applications.
Future Directions:
-
Biosynthetic Engineering: Manipulating the biosynthetic pathway through genetic engineering could enhance mycomycin production, improving the yield and reducing the cost of production.
-
Structure-Activity Relationship (SAR) Studies: Systematically modifying the structure of mycomycin and evaluating the effects on biological activity can guide the design of more potent and less toxic analogues.
-
Drug Delivery Systems: Developing effective drug delivery systems to overcome the instability and toxicity challenges will be crucial for its therapeutic application.
-
Mechanism of Action Studies: Further mechanistic studies are required to understand precisely how mycomycin interacts with its targets and to identify specific interaction sites.
Conclusion: A Promising Antibiotic with Potential
Mycomycin, with its intricate structure and potent biological activity, holds significant promise as a novel antibiotic and potential anticancer agent. While challenges remain in its production, stability, and toxicity profile, ongoing research efforts focused on biosynthetic engineering, SAR studies, and improved drug delivery systems pave the way for harnessing the therapeutic potential of this unique molecule. Further exploration of mycomycin and its analogues may yield valuable new therapies to combat drug-resistant bacteria and other challenging diseases. The continued investigation into its biosynthesis and mechanism of action promises to unravel further the complexity and remarkable properties of this natural wonder.
Latest Posts
Latest Posts
-
Answers To Questions Teachers Ask About Sensory Integration Pdf
Apr 08, 2025
-
Prepare A Cost Of Goods Manufactured Schedule
Apr 08, 2025
-
A Companys Business Model Does Not
Apr 08, 2025
-
T A 1 Of 1 Correr En El Marat A N De Boston
Apr 08, 2025
-
Which Of The Following Is Not True Of Mobile Phones
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Below Is The Structure For The Antibiotic Mycomycin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.