Balance The Equation By Inserting Coefficients As Needed
Holbox
Mar 24, 2025 · 6 min read
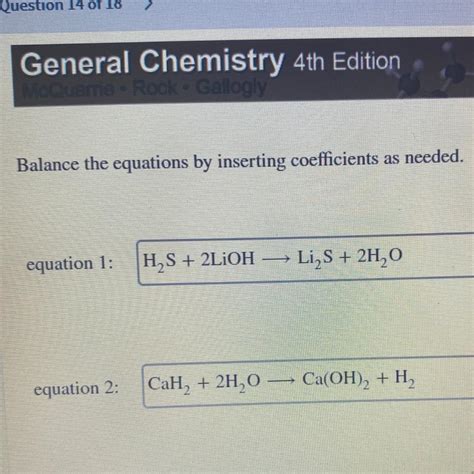
Table of Contents
- Balance The Equation By Inserting Coefficients As Needed
- Table of Contents
- Balancing Chemical Equations: A Comprehensive Guide
- Understanding Chemical Equations
- Methods for Balancing Chemical Equations
- 1. Inspection Method (Trial and Error)
- 2. Algebraic Method
- 3. Oxidation-Reduction (Redox) Method
- Tips for Balancing Chemical Equations
- Common Mistakes to Avoid
- Balancing Equations with Different Reaction Types
- 1. Combustion Reactions:
- 2. Synthesis Reactions (Combination Reactions):
- 3. Decomposition Reactions:
- 4. Single Displacement Reactions:
- 5. Double Displacement Reactions (Metathesis Reactions):
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Balancing Chemical Equations: A Comprehensive Guide
Balancing chemical equations is a fundamental concept in chemistry. It's the process of ensuring that the number of atoms of each element is the same on both the reactant (left-hand side) and product (right-hand side) sides of a chemical equation. This adheres to the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction; it only changes form. Understanding how to balance equations is crucial for accurate stoichiometric calculations, predicting reaction yields, and understanding chemical processes. This guide will provide a comprehensive walkthrough of various techniques and offer tips for success.
Understanding Chemical Equations
Before diving into balancing, let's solidify our understanding of chemical equations. A chemical equation uses chemical formulas and symbols to represent a chemical reaction. For instance:
Reactants → Products
- Reactants: These are the starting materials involved in the chemical reaction. They are placed on the left-hand side of the equation.
- Products: These are the substances formed as a result of the chemical reaction. They are placed on the right-hand side of the equation.
- Arrow (→): This symbol indicates the direction of the reaction. A double arrow (⇌) signifies a reversible reaction, where the products can react to reform the reactants.
- Coefficients: These are the numbers placed in front of chemical formulas to balance the equation. They represent the relative number of molecules or moles of each substance involved. Crucially, never change the subscripts within a chemical formula as this alters the identity of the compound.
Methods for Balancing Chemical Equations
Several methods can be used to balance chemical equations. The best method often depends on the complexity of the equation. Here are some commonly used techniques:
1. Inspection Method (Trial and Error)
This is the simplest method, particularly useful for relatively straightforward equations. It involves systematically adjusting the coefficients until the number of atoms of each element is equal on both sides.
Example: Balance the equation for the combustion of methane:
CH₄ + O₂ → CO₂ + H₂O
-
Start with an element that appears in only one reactant and one product. Let's begin with carbon (C). There's one carbon atom on each side, so carbon is already balanced.
-
Next, balance hydrogen (H). There are four hydrogen atoms on the reactant side (CH₄) and two on the product side (H₂O). To balance, place a coefficient of 2 in front of H₂O:
CH₄ + O₂ → CO₂ + 2H₂O
- Now balance oxygen (O). There are two oxygen atoms on the reactant side and four on the product side (two from CO₂ and two from 2H₂O). To balance, place a coefficient of 2 in front of O₂:
CH₄ + 2O₂ → CO₂ + 2H₂O
Now the equation is balanced. There are one carbon atom, four hydrogen atoms, and four oxygen atoms on both sides.
2. Algebraic Method
This method is more systematic and helpful for complex equations. It involves assigning variables as coefficients and solving a system of algebraic equations.
Example: Balance the equation:
Fe₂O₃ + CO → Fe + CO₂
- Assign variables as coefficients:
aFe₂O₃ + bCO → cFe + dCO₂
- Set up equations based on the number of atoms of each element:
- Fe: 2a = c
- O: 3a + b = 2d
- C: b = d
- Solve the system of equations. This often involves substitution or elimination. Let's choose a = 1 for simplicity. This gives:
- c = 2
- b = d
- 3(1) + b = 2d => 3 + b = 2d
Substituting b = d, we get:
3 + d = 2d => d = 3
Therefore, b = 3.
- Substitute the solved variables back into the equation:
Fe₂O₃ + 3CO → 2Fe + 3CO₂
The equation is now balanced.
3. Oxidation-Reduction (Redox) Method
This method is specifically used for balancing redox reactions, where electrons are transferred between reactants. It involves balancing the oxidation and reduction half-reactions separately before combining them. This is a more advanced technique and involves concepts beyond the scope of a basic balancing guide.
Tips for Balancing Chemical Equations
- Start with the most complex molecule: Balancing the most complex molecule first often simplifies the process.
- Balance polyatomic ions as a unit: If polyatomic ions (like SO₄²⁻ or NO₃⁻) remain unchanged throughout the reaction, treat them as a single unit when balancing.
- Check your work: After balancing, always double-check that the number of atoms of each element is the same on both sides.
- Practice regularly: Balancing chemical equations becomes easier with practice. The more you practice, the more efficient you'll become.
- Use online balancers (with caution): Numerous online tools can balance equations automatically. However, it's crucial to understand the underlying principles and not rely solely on these tools. Use them to verify your work, not replace your understanding.
Common Mistakes to Avoid
- Changing subscripts: Never change the subscripts within a chemical formula. This alters the chemical identity of the substance.
- Forgetting to balance all elements: Make sure you balance every element present in the equation.
- Incorrectly applying coefficients: Coefficients multiply everything in the chemical formula they precede.
- Neglecting to check your work: Always double-check your balanced equation to ensure accuracy.
Balancing Equations with Different Reaction Types
Balancing equations becomes slightly more complex with different reaction types. Let's explore a few examples:
1. Combustion Reactions:
These reactions involve a substance reacting rapidly with oxygen, often producing heat and light. Balancing combustion reactions usually requires careful attention to oxygen atoms. For example, the combustion of propane (C₃H₈):
C₃H₈ + O₂ → CO₂ + H₂O
(The balanced equation is: C₃H₈ + 5O₂ → 3CO₂ + 4H₂O)
2. Synthesis Reactions (Combination Reactions):
These involve two or more reactants combining to form a single product. Balancing these is often straightforward. Example: the formation of water:
H₂ + O₂ → H₂O
(The balanced equation is: 2H₂ + O₂ → 2H₂O)
3. Decomposition Reactions:
These reactions involve a single reactant breaking down into two or more simpler products. Similar to synthesis reactions, balancing is usually straightforward. Example: the decomposition of water:
H₂O → H₂ + O₂
(The balanced equation is: 2H₂O → 2H₂ + O₂ )
4. Single Displacement Reactions:
These reactions involve one element replacing another in a compound. Balancing these can involve more steps. Example:
Zn + HCl → ZnCl₂ + H₂
(The balanced equation is: Zn + 2HCl → ZnCl₂ + H₂)
5. Double Displacement Reactions (Metathesis Reactions):
These reactions involve the exchange of ions between two compounds. Balancing these can be relatively straightforward. Example:
AgNO₃ + NaCl → AgCl + NaNO₃
(This equation is already balanced.)
Conclusion
Balancing chemical equations is a fundamental skill in chemistry. While the inspection method is sufficient for simpler equations, mastering the algebraic method provides a more systematic approach for complex reactions. Remember to always adhere to the law of conservation of mass and to carefully check your work to ensure accuracy. With practice and a clear understanding of the techniques outlined in this guide, you'll confidently balance chemical equations of varying complexities. The ability to do so is a cornerstone of understanding chemical reactions and performing stoichiometric calculations.
Latest Posts
Latest Posts
-
Which Of The Following Solutions Is Basic
Mar 27, 2025
-
The Electrophilic Aromatic Substitution Of Isopropylbenzene With Br2 And Febr3
Mar 27, 2025
-
The Allowance For Sales Discounts Account
Mar 27, 2025
-
Draw The Lewis Structure For Cocl2 Including Lone Pairs
Mar 27, 2025
-
A Bird Watcher Meanders Through The Woods
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Balance The Equation By Inserting Coefficients As Needed . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
