Are The Substances Shown In Italics Undergoing Oxidation Or Reduction
Holbox
Mar 24, 2025 · 5 min read
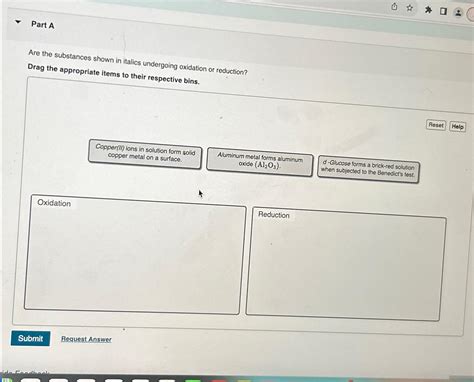
Table of Contents
- Are The Substances Shown In Italics Undergoing Oxidation Or Reduction
- Table of Contents
- Are the Substances Shown in Italics Undergoing Oxidation or Reduction? A Comprehensive Guide
- Understanding Oxidation and Reduction: The Basics
- Identifying Oxidation and Reduction: A Step-by-Step Approach
- Analyzing Example Reactions
- Beyond Simple Examples: Complex Redox Reactions
- Applications of Redox Reactions
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Are the Substances Shown in Italics Undergoing Oxidation or Reduction? A Comprehensive Guide
Determining whether a substance is undergoing oxidation or reduction requires a fundamental understanding of redox reactions. Redox, a shortened form of reduction-oxidation, describes chemical reactions where electrons are transferred between species. One species loses electrons (oxidation), while another gains them (reduction). This process is crucial in numerous chemical and biological processes, from combustion to cellular respiration. This article will delve into the intricacies of redox reactions, providing a clear methodology for identifying oxidation and reduction in various chemical scenarios. We will analyze several examples, systematically determining whether the italicized substances are undergoing oxidation or reduction.
Understanding Oxidation and Reduction: The Basics
The core principle to remember is the mnemonic OIL RIG: Oxidation Is Loss (of electrons), and Reduction Is Gain (of electrons).
-
Oxidation: A substance is oxidized when it loses electrons. This often involves an increase in oxidation state (a number assigned to an atom in a molecule reflecting its apparent charge). Oxidation is frequently accompanied by an increase in the number of bonds to oxygen or a decrease in the number of bonds to hydrogen.
-
Reduction: A substance is reduced when it gains electrons. This results in a decrease in oxidation state. Reduction is often associated with a decrease in the number of bonds to oxygen or an increase in the number of bonds to hydrogen.
Example: Consider the simple reaction:
2Na + Cl₂ → 2NaCl
- Sodium (Na) loses one electron to become Na⁺ (oxidation).
- Chlorine (Cl₂) gains one electron per atom to become Cl⁻ (reduction).
Identifying Oxidation and Reduction: A Step-by-Step Approach
To determine whether a substance is undergoing oxidation or reduction, follow these steps:
-
Assign Oxidation States: Assign oxidation states to all atoms in the reactants and products. This involves applying a set of rules, including the oxidation state of an element in its elemental form being zero, and the oxidation state of monatomic ions being equal to their charge.
-
Compare Oxidation States: Compare the oxidation states of the italicized substance in the reactants and products.
-
Determine Electron Transfer: If the oxidation state of the italicized substance increases, it has lost electrons and is undergoing oxidation. If the oxidation state decreases, it has gained electrons and is undergoing reduction.
Analyzing Example Reactions
Let's analyze several example reactions, focusing on whether the substances in italics are being oxidized or reduced:
Example 1:
Fe²⁺ + Cu²⁺ → Fe³⁺ + Cu⁺
-
Oxidation States: Fe²⁺ (oxidation state +2), Cu²⁺ (+2), Fe³⁺ (+3), Cu⁺ (+1).
-
Comparison: The oxidation state of iron increases from +2 to +3.
-
Conclusion: Iron is undergoing oxidation because it loses an electron. Copper is simultaneously undergoing reduction.
Example 2:
2H₂ + O₂ → 2H₂O
-
Oxidation States: H₂ (oxidation state 0), O₂ (0), H in H₂O (+1), O in H₂O (-2).
-
Comparison: The oxidation state of hydrogen increases from 0 to +1.
-
Conclusion: Hydrogen is undergoing oxidation. Oxygen is undergoing reduction.
Example 3:
MnO₄⁻ + 8H⁺ + 5e⁻ → Mn²⁺ + 4H₂O
-
Oxidation States: Mn in MnO₄⁻ (+7), Mn²⁺ (+2), H⁺ (+1), H in H₂O (+1), O in H₂O (-2).
-
Comparison: The oxidation state of manganese decreases from +7 to +2.
-
Conclusion: Manganese is undergoing reduction. It gains five electrons.
Example 4:
Zn + Cu²⁺ → Zn²⁺ + Cu
-
Oxidation States: Zn (0), Cu²⁺ (+2), Zn²⁺ (+2), Cu (0).
-
Comparison: The oxidation state of copper decreases from +2 to 0.
-
Conclusion: Copper is undergoing reduction. Zinc is simultaneously undergoing oxidation.
Example 5: A more complex organic example: The oxidation of ethanol to ethanal.
CH₃CH₂OH → CH₃CHO + 2H⁺ + 2e⁻
-
Oxidation States: Determining oxidation states in organic molecules can be more complex, often requiring a detailed analysis of the bonds. However, we can focus on the carbon atom bonded to the hydroxyl group. In ethanol, this carbon is less oxidized (more bonds to hydrogen). In ethanal, this carbon has one less bond to hydrogen and one more bond to oxygen – a characteristic of oxidation.
-
Comparison: The carbon atom in ethanol loses hydrogen atoms and gains a bond to oxygen. This reflects an increase in the oxidation state.
-
Conclusion: Ethanol is undergoing oxidation.
Example 6: The reduction of a carbonyl group to an alcohol.
CH₃CHO + 2H⁺ + 2e⁻ → CH₃CH₂OH
-
Oxidation States: Using the same principle as above, the carbonyl carbon in ethanal has a higher oxidation state than the carbon in ethanol due to the double bond to oxygen.
-
Comparison: The carbon atom in the ethanal gains hydrogen atoms, decreasing its oxidation state.
-
Conclusion: Ethanal is undergoing reduction.
Beyond Simple Examples: Complex Redox Reactions
Many redox reactions are significantly more complex than the simple examples shown above. They may involve multiple steps, changes in multiple elements, or intricate organic structures. However, the fundamental principles of electron transfer and changes in oxidation states remain the cornerstone for understanding these processes. For complex reactions, the use of half-reactions (separating the oxidation and reduction processes) can be particularly helpful in elucidating the electron transfer occurring.
Applications of Redox Reactions
Redox reactions are ubiquitous in nature and have a vast array of applications in various fields:
-
Biology: Cellular respiration, photosynthesis, and many metabolic processes rely on redox reactions. Electron transport chains are critical for energy production in living organisms.
-
Industry: Metallurgy (extraction of metals from ores), battery technology, and various industrial chemical processes utilize redox reactions extensively.
-
Environmental Science: Redox reactions play a role in water purification, soil remediation, and the cycling of nutrients in ecosystems.
Understanding redox reactions is therefore essential for a comprehensive grasp of numerous scientific and technological disciplines.
Conclusion
Determining whether a substance is undergoing oxidation or reduction necessitates a systematic approach involving the assignment and comparison of oxidation states. By applying the principles outlined in this article, one can accurately identify the oxidation or reduction of substances in a wide array of chemical reactions, from simple ionic equations to complex organic transformations. Remembering the OIL RIG mnemonic and systematically analyzing oxidation state changes are key to mastering this fundamental concept in chemistry. Further practice with diverse examples will solidify your understanding and ability to analyze redox processes with confidence.
Latest Posts
Latest Posts
-
You Re Doing On Page Search Engine
Mar 27, 2025
-
Do Black People Have An Extra Calf Muscle
Mar 27, 2025
-
Determine The Magnitude Of The Pin Force At A
Mar 27, 2025
-
What Is The Advantage Of Using A Wet Mount
Mar 27, 2025
-
What Is The Name Of The Following
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Are The Substances Shown In Italics Undergoing Oxidation Or Reduction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
