All Of The Following Are Representations Of 2-methylpentane Except
Holbox
Mar 21, 2025 · 5 min read
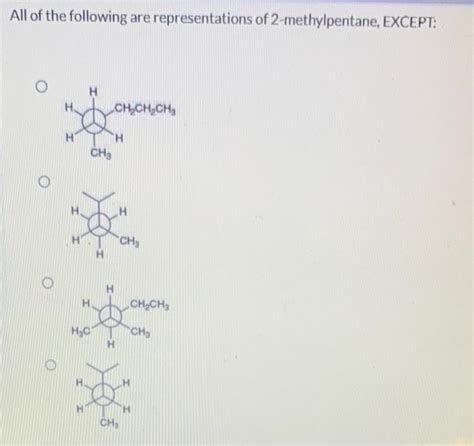
Table of Contents
All of the Following are Representations of 2-Methylpentane Except…
Understanding organic chemistry, specifically the representation of branched alkanes like 2-methylpentane, requires a keen eye for detail. The seemingly simple molecule can be depicted in numerous ways, from condensed formulas to complex 3D models. This article will delve into the various ways 2-methylpentane can be represented, highlighting the subtle differences that can lead to misidentification. We'll explore common representations, focusing on what constitutes a correct depiction and what constitutes an incorrect one. By the end, you will be able to confidently identify accurate and inaccurate representations of 2-methylpentane.
Understanding 2-Methylpentane
Before we dissect different representations, let's establish a firm understanding of 2-methylpentane itself. It's an isomer of heptane (C₇H₁₆), meaning it has the same molecular formula but a different structural arrangement. The "pentane" part indicates a five-carbon chain as the parent structure. The "2-methyl" prefix signifies a methyl group (CH₃) attached to the second carbon atom in that chain.
Common Representations of 2-Methylpentane
2-Methylpentane can be represented in several ways, each with its own advantages and disadvantages in terms of clarity and ease of understanding. Let's explore the most common ones:
1. Condensed Structural Formula
This representation shows all the atoms and their connections, but omits explicit depiction of all the bonds. It's a compact way to represent the molecule. For 2-methylpentane, a common condensed structural formula would be:
CH₃CH₂CH(CH₃)CH₂CH₃
This clearly shows the five-carbon chain and the methyl group on the second carbon.
2. Skeletal Formula (Line-Angle Formula)
This is a simplified representation where carbon atoms are implied at the corners and ends of lines, and hydrogen atoms are omitted. Only heteroatoms (atoms other than carbon and hydrogen) are explicitly shown. For 2-methylpentane, the skeletal formula is:
CH3
|
CH3-CH-CH2-CH2-CH3
This is a very efficient way to represent the molecule, particularly useful for larger and more complex structures.
3. Expanded Structural Formula
This representation shows every atom and every bond explicitly. It's the most detailed representation and is helpful for visualizing the three-dimensional structure. For 2-methylpentane, it would look like this:
H H H H
| | | |
H-C-C-C-C-C-H
| | | |
H H CH3 H
While detailed, this representation can become cumbersome for larger molecules.
4. 3D Models (Ball-and-Stick and Space-Filling)
These models offer the most realistic depiction of the molecule's three-dimensional structure. Ball-and-stick models show atoms as balls and bonds as sticks, while space-filling models show atoms as spheres with radii proportional to their van der Waals radii, giving a better representation of the molecule's actual size and shape. These models are particularly useful for visualizing steric hindrance and molecular interactions.
Representations that are NOT 2-Methylpentane
Now let's look at some examples of structures that are not 2-methylpentane, highlighting why they are incorrect:
1. 3-Methylpentane
This isomer has the methyl group attached to the third carbon atom of the pentane chain. Its condensed formula is CH₃CH₂CH₂CH(CH₃)CH₃, distinctly different from 2-methylpentane.
2. Hexane
Hexane (C₆H₁₄) has a six-carbon chain and lacks the methyl group. Its condensed formula is CH₃CH₂CH₂CH₂CH₂CH₃. It's a completely different alkane altogether.
3. 2,2-Dimethylbutane
This molecule has a four-carbon chain with two methyl groups on the second carbon. Its condensed formula is CH₃C(CH₃)₂CH₂CH₃. Although it has the same molecular formula (C₆H₁₄), the branching is different.
4. Incorrect Skeletal Structures
Even in skeletal formulas, errors can occur. For instance, a structure that shows the methyl group attached to the wrong carbon or incorrectly represents the carbon chain length would not be 2-methylpentane.
5. Incorrect Condensed Structures
Similar to skeletal structures, errors in the condensed formula, such as incorrect placement of parentheses or the number of carbons and hydrogens, will result in a different molecule.
Identifying Incorrect Representations: A Systematic Approach
To avoid errors in identifying 2-methylpentane, follow these steps:
- Count the carbons: Ensure there are six carbon atoms in total.
- Identify the parent chain: Confirm the presence of a five-carbon chain (pentane).
- Locate the methyl group: Verify that a methyl group (CH₃) is attached to the second carbon of the pentane chain.
- Check for other substituents: Make sure there are no other substituents (branches or functional groups) present.
- Consider isomers: Be aware of the possibility of other isomers (like 3-methylpentane) which share the same molecular formula but have different structures.
Advanced Considerations: Conformational Isomers
While the previous examples focus on constitutional isomers (molecules with the same formula but different connectivity), 2-methylpentane also exhibits conformational isomers (different spatial arrangements due to rotation around single bonds). These different conformations do not represent different compounds, but rather different energy states of the same molecule. Understanding this distinction is important for advanced organic chemistry.
Conclusion: Mastering 2-Methylpentane Representations
Mastering the representation of 2-methylpentane and distinguishing it from other molecules requires a thorough understanding of organic chemistry nomenclature and structural conventions. By carefully following the systematic approach outlined above and practicing with different representations, you can confidently identify accurate and inaccurate depictions of 2-methylpentane. Remember to pay close attention to the number of carbon atoms, the parent chain length, the position of substituents, and the overall connectivity. Consistent practice is key to solidifying this knowledge and avoiding common pitfalls. The ability to correctly interpret these representations is crucial for success in organic chemistry and related fields.
Latest Posts
Latest Posts
-
Business Communication Developing Leaders For A Networked World
Mar 21, 2025
-
Apply A Top And Double Bottom Border To Range A12 D12
Mar 21, 2025
-
Production Order Processing Is An Example Of A
Mar 21, 2025
-
Which Of The Following Is True About An Unmanaged Switch
Mar 21, 2025
-
Most Ethical Codes Specify That Therapists Should
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about All Of The Following Are Representations Of 2-methylpentane Except . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
