A Solution Of Kcl Is Saturated At 50 C
Holbox
Mar 17, 2025 · 6 min read
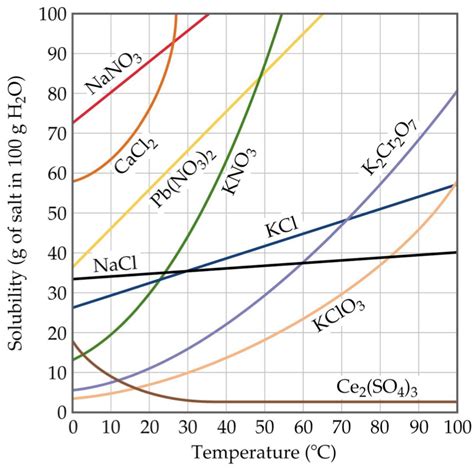
Table of Contents
A Solution of KCl is Saturated at 50°C: Understanding Solubility and its Implications
Solubility, a fundamental concept in chemistry, refers to the maximum amount of a solute that can dissolve in a given amount of solvent at a specific temperature and pressure. Understanding solubility is crucial in various fields, from chemical engineering and pharmaceuticals to environmental science and geology. This article delves into the specifics of a potassium chloride (KCl) solution saturated at 50°C, exploring the factors influencing solubility, the implications of saturation, and practical applications.
What is a Saturated Solution?
A saturated solution is one in which no more solute can dissolve at a given temperature and pressure. Any additional solute added will simply settle at the bottom, remaining undissolved. This equilibrium point is dictated by the inherent properties of the solute and solvent, as well as external factors like temperature and pressure. In the case of our KCl solution saturated at 50°C, this means that at this specific temperature, the water has dissolved the maximum possible amount of KCl.
Understanding the Dynamic Equilibrium
It's important to note that saturation doesn't mean the dissolution process has stopped entirely. Instead, it represents a dynamic equilibrium. At the saturation point, the rate at which KCl dissolves is equal to the rate at which it precipitates out of the solution. KCl ions are constantly moving between the dissolved and undissolved states, maintaining a constant concentration.
Factors Affecting KCl Solubility
Several factors influence the solubility of KCl in water, and therefore the point at which a solution becomes saturated at 50°C. These include:
1. Temperature: The Dominant Factor
Temperature is the most significant factor affecting the solubility of most solids in liquids, including KCl. Generally, the solubility of solids increases with increasing temperature. This is because higher temperatures provide the dissolved particles with more kinetic energy, allowing them to overcome the intermolecular forces holding the crystal lattice together. For KCl, the solubility increases noticeably as the temperature rises, meaning a significantly larger amount of KCl can dissolve in water at 50°C compared to, say, 25°C.
2. Pressure: A Minor Influence
Pressure typically has a negligible effect on the solubility of solids in liquids. Unlike gases, whose solubility is strongly influenced by pressure, the volume changes associated with dissolving solids are minimal. Therefore, changes in pressure will not significantly alter the saturation point of our KCl solution at 50°C.
3. Nature of the Solvent: Polarity Matters
The nature of the solvent plays a crucial role in solubility. "Like dissolves like" is a fundamental principle. Polar solvents, like water, dissolve polar solutes effectively. KCl is an ionic compound, composed of K⁺ and Cl⁻ ions, making it a polar solute. Water, being a highly polar solvent, readily interacts with these ions through dipole-dipole interactions and ion-dipole interactions, facilitating dissolution. Non-polar solvents, on the other hand, would have significantly lower solubility for KCl.
4. Presence of Other Solutes: The Common Ion Effect
The presence of other solutes in the solution can also affect KCl solubility. The common ion effect is a prime example. If other potassium salts (like KNO₃) or chloride salts (like NaCl) are present in the solution, the solubility of KCl will decrease. This is because the increased concentration of either K⁺ or Cl⁻ ions shifts the equilibrium towards the precipitation of KCl, reducing the amount of KCl that can remain dissolved.
Implications of a Saturated KCl Solution at 50°C
Understanding that a KCl solution is saturated at 50°C has several practical implications:
1. Crystallization: Obtaining Pure KCl
When a saturated KCl solution at 50°C is cooled, its solubility decreases. This decrease in solubility leads to crystallization, where excess KCl precipitates out of the solution, forming pure KCl crystals. This process is frequently used in the purification of KCl from impure samples.
2. Precipitation Reactions: Selective Separation
The knowledge of saturation at a specific temperature allows for the controlled precipitation of KCl in chemical reactions. By carefully manipulating the temperature and concentration of reactants, one can selectively precipitate KCl, separating it from other dissolved components.
3. Electrolyte Solutions: Conductivity and Applications
Saturated KCl solutions find applications as electrolyte solutions in various electrochemical processes. The high concentration of ions in a saturated solution ensures high electrical conductivity, which is essential for applications such as electrochemical cells, conductivity measurements, and reference electrodes.
4. Agricultural Applications: Fertilizers
Potassium is an essential nutrient for plant growth, and KCl is a major component of many potassium fertilizers. Understanding the solubility of KCl helps determine the optimal concentration for fertilizer solutions, ensuring effective nutrient uptake by plants.
5. Medical Applications: Intravenous Fluids and Other Applications
KCl is an essential electrolyte in the human body and is used in intravenous fluids to maintain electrolyte balance in patients. Precise control over KCl concentration is crucial to avoid adverse effects, making an understanding of saturation and solubility paramount.
Determining the Saturation Point Experimentally
The exact solubility of KCl at 50°C can be determined experimentally through a simple procedure:
- Preparation: Start with a known amount of distilled water.
- Addition of KCl: Gradually add KCl to the water while stirring continuously.
- Saturation Point: Continue adding KCl until no more dissolves, and undissolved KCl remains at the bottom.
- Filtration: Filter the solution to remove the undissolved KCl.
- Determination: Carefully determine the mass of the dissolved KCl and the volume of the water. Calculate the solubility as grams of KCl per 100 mL of water.
- Temperature Control: Maintain the temperature at 50°C throughout the experiment using a water bath or other temperature-control device.
Beyond 50°C: Exploring Solubility Curves
The solubility of KCl at 50°C is just one point on its solubility curve. A solubility curve is a graphical representation of the solubility of a substance as a function of temperature. Constructing and analyzing solubility curves for KCl (and other substances) provides a comprehensive understanding of their solubility behavior over a range of temperatures. This information is invaluable for various applications, including crystallization, purification, and process optimization.
Conclusion: The Significance of Solubility
The saturation of a KCl solution at 50°C is a seemingly simple observation, yet it encapsulates fundamental principles of chemistry and has far-reaching practical implications. Understanding solubility, its dependence on temperature and other factors, and the concept of dynamic equilibrium is crucial for numerous scientific disciplines and industrial applications. From fertilizer production to intravenous fluid preparation, the ability to control and predict the solubility of KCl is essential for achieving optimal results and ensuring safety. Further exploration of solubility curves and the effects of other factors will continue to enhance our understanding and utilization of this fundamental chemical property. This detailed knowledge is vital for developing innovative solutions and improving existing processes across a wide range of fields.
Latest Posts
Latest Posts
-
Endocytosis Moves Materials A Cell Via
Mar 18, 2025
-
Behaviorism Focuses On Making Psychology An Objective Science By
Mar 18, 2025
-
How To Link Chegg To Tinder
Mar 18, 2025
-
The Nucleus Of An Atom Contains
Mar 18, 2025
-
What Sign Of Cockroach Infestation Might Food Workers Notice
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about A Solution Of Kcl Is Saturated At 50 C . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
