A Red Blood Cell Placed In A Hypertonic Medium Will
Holbox
Mar 12, 2025 · 5 min read
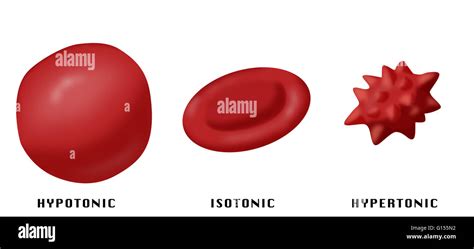
Table of Contents
A Red Blood Cell Placed in a Hypertonic Medium Will: Understanding Osmosis and its Effects
Understanding the behavior of cells in different environments is fundamental to biology. One crucial concept is osmosis, the movement of water across a selectively permeable membrane from an area of high water concentration to an area of low water concentration. This movement aims to equalize the concentration of solutes on both sides of the membrane. Let's explore what happens when a red blood cell, or erythrocyte, is placed in a hypertonic medium.
What is a Hypertonic Solution?
A hypertonic solution is one that has a higher concentration of solutes (dissolved substances) compared to another solution, separated by a semipermeable membrane. Think of it like this: if you have a glass of saltwater and a glass of pure water, the saltwater is hypertonic compared to the pure water. The higher concentration of salt (the solute) means there's less water relative to the solutes.
What Happens to a Red Blood Cell in a Hypertonic Solution?
When a red blood cell is placed in a hypertonic solution, water will move out of the cell and into the surrounding solution. This is because the concentration of solutes is higher outside the cell than inside. The cell's membrane, while selectively permeable, allows water to pass through, but not the solutes. This outward movement of water causes the cell to shrink and crenate.
Crenation, also known as plasmolysis in plant cells, is the process where the cell membrane pulls away from the cell wall due to water loss. In red blood cells, lacking a rigid cell wall, this results in a shrunken, spiky, or irregularly shaped cell. The extent of crenation depends on the degree of hypertonicity (how much higher the solute concentration is outside the cell) and the duration of exposure.
The Role of the Cell Membrane
The cell membrane, or plasma membrane, plays a vital role in this process. It's a selectively permeable barrier, meaning it controls which substances can pass through. Water molecules, being small and uncharged, can pass through the membrane via osmosis. However, larger molecules and charged ions generally cannot pass freely. This selective permeability ensures that the cell can maintain its internal environment distinct from its surroundings.
The Osmotic Pressure Gradient
The driving force behind water movement in this scenario is the osmotic pressure gradient. The greater the difference in solute concentration between the inside and outside of the cell, the steeper the gradient and the faster the water moves. This pressure is essentially the force exerted by water molecules as they try to equalize the concentration of solutes.
Consequences of Crenation
The crenation of red blood cells due to exposure to a hypertonic solution can have serious consequences. These shrunken cells may:
- Lose their flexibility: This reduces their ability to navigate through narrow capillaries, impeding oxygen delivery to tissues.
- Become dysfunctional: Their altered shape interferes with their normal function of carrying oxygen.
- Undergo hemolysis: While crenation is primarily a consequence of water loss, severe hypertonicity can also trigger hemolysis (rupture of red blood cells) if the osmotic pressure gradient is extremely high. This releases hemoglobin into the plasma, potentially leading to serious health problems.
Practical Examples of Hypertonic Environments
Understanding the effects of hypertonic solutions on red blood cells has several practical implications in different contexts:
-
Dehydration: When the body is dehydrated, the concentration of solutes in the blood plasma increases, making it hypertonic relative to the red blood cells. This leads to water moving out of the red blood cells, causing them to crenate. Severe dehydration can compromise oxygen delivery and potentially cause health issues.
-
Intravenous Fluid Therapy: Clinicians carefully select intravenous fluids to avoid harming red blood cells. Isotonic solutions (having the same solute concentration as the cells) are preferred to prevent crenation or lysis (bursting) of the red blood cells. Administering a hypertonic solution intravenously can lead to potentially dangerous complications.
-
Food Preservation: High concentrations of salt or sugar are often used to preserve food. These hypertonic environments draw water out of microorganisms, inhibiting their growth and preventing spoilage. This principle is applied in techniques like salting meat or making jams and jellies.
-
Medical Treatments: Some medical treatments utilize hypertonic solutions, but this requires careful control and monitoring to avoid damaging red blood cells. The specific concentration and duration of exposure must be carefully considered to achieve the therapeutic effect while minimizing the adverse effects on red blood cells and other cells.
Comparing Hypertonic Solutions to Other Solutions
To better understand hypertonicity, it's helpful to compare it to other types of solutions:
-
Isotonic Solutions: In an isotonic solution, the concentration of solutes is equal inside and outside the cell. There's no net movement of water, and the cell maintains its normal shape and function. This is the ideal environment for red blood cells.
-
Hypotonic Solutions: In a hypotonic solution, the concentration of solutes is lower outside the cell than inside. Water moves into the cell, causing it to swell and potentially burst (lyse).
Cellular Response and Mechanisms
The response of a red blood cell to a hypertonic environment is not passive; it involves active cellular mechanisms:
-
Aquaporins: These are water channels embedded in the cell membrane that facilitate the rapid movement of water across the membrane. In hypertonic conditions, water flows out through aquaporins, accelerating the crenation process.
-
Membrane Proteins: These proteins help maintain the cell's structure and integrity. In hypertonic conditions, these proteins undergo changes to attempt to compensate for the water loss and maintain the cell's shape as much as possible. However, extreme hypertonicity will overcome these compensatory mechanisms.
-
Cellular Signaling: The cell senses changes in its internal environment due to water loss. This triggers cellular signaling pathways that attempt to regulate the situation, but if the hypertonicity is significant, these pathways might not be sufficient to prevent crenation.
Conclusion: The Importance of Osmosis and Homeostasis
The fate of a red blood cell placed in a hypertonic medium highlights the crucial role of osmosis in maintaining cellular homeostasis. The ability of cells to regulate their water content is essential for their proper functioning. Understanding these principles is vital in various fields, from medicine and physiology to food science and environmental biology. Disruptions to osmotic balance can have significant consequences for cellular health and overall organismal health. Maintaining an isotonic environment for red blood cells is critical for efficient oxygen transport and overall well-being. The detailed understanding of crenation and the mechanisms involved underscores the complexity and delicate balance required for maintaining cellular life.
Latest Posts
Latest Posts
-
Meaningfulness Is Associated With Blank Rather Than Blank
Mar 12, 2025
-
Locking Out Tagging Out Refers To The Practice Of
Mar 12, 2025
-
What Is The Value Of The
Mar 12, 2025
-
You Open A Document To Find The Text Illegible
Mar 12, 2025
-
On July 1 A Company Receives An Invoice For 800
Mar 12, 2025
Related Post
Thank you for visiting our website which covers about A Red Blood Cell Placed In A Hypertonic Medium Will . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
