4 Isopropyl 2 4 5 Trimethylheptane
Holbox
Mar 15, 2025 · 6 min read
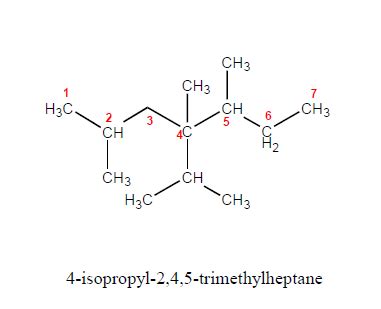
Table of Contents
Decoding 4-Isopropyl-2,4,5-trimethylheptane: A Deep Dive into its Structure, Properties, and Applications
4-Isopropyl-2,4,5-trimethylheptane, a complex branched alkane, presents a fascinating case study in organic chemistry. Its intricate structure leads to unique properties and potential applications, though currently, its specific uses are limited due to its relative obscurity and the challenges in its synthesis. This article delves deep into the chemical characteristics of this compound, exploring its structural analysis, predicted properties, potential synthesis routes, and its place within the broader context of organic chemistry.
Understanding the IUPAC Nomenclature
The name itself, 4-isopropyl-2,4,5-trimethylheptane, is a testament to the systematic nomenclature used in organic chemistry. Let's break it down:
- Heptane: This indicates a seven-carbon alkane chain as the parent structure. Think of it as the backbone of the molecule.
- Tri-methyl: This signifies the presence of three methyl (CH3) groups attached to the heptane chain.
- 2,4,5-: These numbers specify the location of the three methyl groups on the heptane carbon chain. The numbering starts from the end of the chain that gives the lowest possible numbers to the substituents.
- Isopropyl: This indicates an isopropyl group, a branched alkyl group with the formula -CH(CH3)2, attached to the heptane chain.
- 4-: This number specifies the location of the isopropyl group on the heptane carbon chain.
This meticulous naming convention allows chemists worldwide to unambiguously identify and understand the specific structure of this complex molecule. The precise arrangement of the substituents is crucial, as even slight changes in position can drastically alter the compound's properties.
Structural Elucidation and Isomerism
The structure of 4-isopropyl-2,4,5-trimethylheptane can be visualized as a seven-carbon chain with multiple branches. Constructing a skeletal structure is often the best method to understand this.
Visualizing the Structure: Imagine a straight line representing the seven-carbon heptane chain. Then, add the three methyl groups at carbons 2, 4, and 5 and the isopropyl group at carbon 4. This results in a highly branched structure with a significant degree of steric hindrance – meaning the bulky groups hinder each other spatially.
Isomerism: It's important to note that many isomers are possible for a molecule with this formula. Isomers are molecules with the same chemical formula but different arrangements of atoms. The specific arrangement dictated by the IUPAC name is crucial to distinguish it from its numerous isomers. Subtle changes in the position of the methyl and isopropyl groups would create different molecules with potentially vastly different properties. Determining the precise isomer is critical for any discussion of its properties or applications.
Predicted Physical and Chemical Properties
Due to the lack of readily available experimental data on 4-isopropyl-2,4,5-trimethylheptane, predicting its properties requires a combination of theoretical calculations and comparisons with similar branched alkanes.
- State of Matter: Given its molecular weight and structure, it's highly likely to exist as a colorless liquid at room temperature.
- Solubility: Being a non-polar hydrocarbon, it is expected to be insoluble in water but soluble in many organic solvents like hexane, benzene, and chloroform.
- Boiling Point: The extensive branching significantly impacts the boiling point. Compared to a straight-chain heptane, the high degree of branching reduces the surface area for intermolecular forces, leading to a lower boiling point. Precise prediction requires computational chemistry techniques.
- Density: Similarly, the density is expected to be slightly lower than that of straight-chain alkanes due to the branched structure.
- Flammability: Like most alkanes, it's expected to be highly flammable, reacting readily with oxygen to produce carbon dioxide and water.
- Reactivity: It's relatively unreactive compared to other functionalized organic molecules. Typical alkane reactions, such as combustion and halogenation, are expected. However, the steric hindrance from the branching could influence the reaction rates and selectivity.
Potential Synthesis Routes
Synthesizing 4-isopropyl-2,4,5-trimethylheptane is likely challenging due to the complex branching. Several synthetic routes could potentially be explored, though each would require careful optimization:
-
Grignard Reactions: A multi-step synthesis using Grignard reagents could be employed. This would involve sequential addition of appropriate alkyl magnesium halides to a suitable ketone or aldehyde precursor. The branching would necessitate careful control of reaction conditions to minimize side reactions and achieve high selectivity.
-
Wurtz Coupling: This method involves coupling alkyl halides using metallic sodium. However, achieving the specific branched structure requires selecting the correct starting materials and precise reaction conditions. The challenge lies in controlling the selectivity to obtain the desired isomer.
-
Alkylation of Alkanes: This approach could involve alkylating a suitable alkane precursor with appropriate alkyl halides in the presence of a strong Lewis acid catalyst. Again, controlling the selectivity to produce the desired isomer would be a significant challenge.
Regardless of the chosen method, purification of the final product would be crucial, likely requiring techniques like fractional distillation and chromatography to isolate the desired isomer from any side products.
Potential Applications (Speculative)
Currently, there are no documented specific applications for 4-isopropyl-2,4,5-trimethylheptane. However, given its properties, some speculative applications can be considered:
- Solvent: Its non-polar nature and solubility in organic solvents could potentially make it a solvent for specific applications. However, its synthesis complexity would likely make this economically unfeasible compared to more readily available solvents.
- Fuel Additive: Its high energy content could make it a potential component in fuel blends. However, the branched structure might affect its combustion efficiency, and further research is needed to assess its feasibility.
- Chemical Intermediate: It could potentially serve as an intermediate in the synthesis of more complex molecules, though this would require further research to explore its reactivity and suitability for various reactions.
Conclusion: Future Research and Significance
4-isopropyl-2,4,5-trimethylheptane, despite its relative obscurity, presents an interesting challenge in organic chemistry. Its complex structure offers opportunities to explore advanced synthetic strategies, and a deeper understanding of its properties could reveal unexpected applications. Further research, including experimental determination of its physical and chemical properties, is crucial. Computational chemistry techniques could aid in predicting these properties and designing efficient synthetic routes. Exploring its potential uses as a solvent, fuel additive, or chemical intermediate requires further investigation. Although currently lacking widespread practical applications, this compound provides valuable insights into the intricate world of organic chemistry and serves as a testament to the power of systematic nomenclature in precisely defining complex molecular structures. The continued exploration of this compound and similar highly branched alkanes will undoubtedly contribute to advancements in organic synthesis and potentially lead to novel applications in various fields. Its study highlights the vastness of chemical space and the ongoing challenges and rewards of unraveling the properties and potential of complex organic molecules.
Latest Posts
Latest Posts
-
Can You Highlight In A Chegg Rental
Mar 15, 2025
-
Which Is The Base Peak Chegg
Mar 15, 2025
-
How Long Does Chegg Take To Ship
Mar 15, 2025
-
How To Link Chegg And Tinder
Mar 15, 2025
-
Is Your Nose Getting Bigger Chegg
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about 4 Isopropyl 2 4 5 Trimethylheptane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
