Write The Equation For The Solubility Product For Lead Iodide
Holbox
Mar 28, 2025 · 5 min read
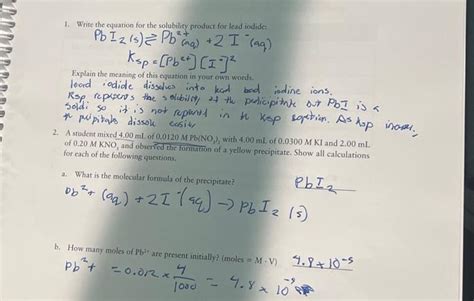
Table of Contents
- Write The Equation For The Solubility Product For Lead Iodide
- Table of Contents
- The Solubility Product Equation for Lead Iodide: A Deep Dive
- Understanding Solubility and Solubility Product (Ksp)
- Calculating Ksp for Lead Iodide
- Factors Affecting the Solubility of Lead Iodide
- 1. Temperature:
- 2. Common Ion Effect:
- 3. Complex Ion Formation:
- 4. pH:
- Applications of Understanding PbI₂ Solubility
- 1. Lead Iodide-Based Solar Cells:
- 2. X-ray and Gamma-ray Detectors:
- 3. Environmental Chemistry:
- 4. Analytical Chemistry:
- Beyond the Basics: Advanced Concepts
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
The Solubility Product Equation for Lead Iodide: A Deep Dive
Lead iodide (PbI₂) is a fascinating compound with applications ranging from solar cells to X-ray detectors. Understanding its solubility and, crucially, its solubility product (Ksp), is key to predicting its behavior in various chemical systems. This article delves into the equation for the solubility product of lead iodide, exploring the concepts behind it and its practical implications. We'll cover the equilibrium expression, how to calculate Ksp, factors affecting solubility, and real-world applications.
Understanding Solubility and Solubility Product (Ksp)
Solubility refers to the maximum amount of a solute that can dissolve in a given amount of solvent at a specific temperature and pressure to form a saturated solution. When an ionic compound like lead iodide dissolves in water, it dissociates into its constituent ions: lead(II) ions (Pb²⁺) and iodide ions (I⁻). This process reaches equilibrium when the rate of dissolution equals the rate of precipitation.
The solubility product constant (Ksp) is an equilibrium constant that represents the product of the concentrations of the ions raised to the power of their stoichiometric coefficients in a saturated solution of a sparingly soluble salt. For lead iodide, the dissolution equilibrium is:
PbI₂(s) ⇌ Pb²⁺(aq) + 2I⁻(aq)
The corresponding solubility product expression is:
Ksp = [Pb²⁺][I⁻]²
This equation tells us that the solubility product is equal to the concentration of lead(II) ions multiplied by the square of the concentration of iodide ions. The square term arises from the stoichiometry of the dissolution reaction; two iodide ions are produced for every one lead(II) ion.
Calculating Ksp for Lead Iodide
Calculating the Ksp value requires knowing the solubility (s) of lead iodide. Solubility is often expressed in moles per liter (mol/L) or molarity (M). Let's assume that the solubility of PbI₂ is 's' mol/L. According to the stoichiometry of the dissolution reaction, when 's' moles of PbI₂ dissolve, 's' moles of Pb²⁺ and 2s moles of I⁻ are produced. Therefore:
- [Pb²⁺] = s
- [I⁻] = 2s
Substituting these values into the Ksp expression:
Ksp = (s)(2s)² = 4s³
This equation provides a direct relationship between the solubility (s) of lead iodide and its solubility product (Ksp). If we know the solubility, we can easily calculate the Ksp, and vice versa. Experimental methods are used to determine the solubility of PbI₂; once the solubility is known, the Ksp can be calculated.
Factors Affecting the Solubility of Lead Iodide
Several factors can influence the solubility of lead iodide and thus its Ksp value:
1. Temperature:
Generally, the solubility of most ionic compounds, including lead iodide, increases with increasing temperature. Higher temperatures provide more kinetic energy to the ions, overcoming the attractive forces holding the solid lattice together. This leads to a higher concentration of ions in the saturated solution and consequently a larger Ksp value.
2. Common Ion Effect:
The presence of a common ion in the solution significantly reduces the solubility of lead iodide. If we add a soluble iodide salt (e.g., KI or NaI) to a saturated solution of PbI₂, the concentration of iodide ions ([I⁻]) increases. According to Le Chatelier's principle, the equilibrium shifts to the left, favoring the precipitation of PbI₂, thus decreasing its solubility. The Ksp remains constant at a given temperature, but the solubility (s) decreases.
3. Complex Ion Formation:
The addition of certain ligands that can form complex ions with Pb²⁺ can enhance the solubility of lead iodide. These ligands compete with iodide ions for lead(II) ions, thereby reducing the concentration of free Pb²⁺ in the solution. This shift in equilibrium again increases the solubility of PbI₂. However, it's important to note this doesn't alter the Ksp value itself; it affects the solubility (s).
4. pH:
The pH of the solution can indirectly affect the solubility of lead iodide. If the pH is highly acidic or basic, it can influence the speciation of lead(II) ions and potentially form other lead complexes. This indirect effect might slightly alter the overall solubility.
Applications of Understanding PbI₂ Solubility
Understanding the solubility and Ksp of lead iodide has various practical applications:
1. Lead Iodide-Based Solar Cells:
Lead iodide is a crucial component in perovskite solar cells. Controlling its solubility and crystallinity during the synthesis process is crucial for optimizing the performance of these cells. Understanding Ksp allows scientists to fine-tune the conditions for creating high-quality perovskite films.
2. X-ray and Gamma-ray Detectors:
Lead iodide is used as a scintillating material in detectors due to its ability to convert X-rays and gamma rays into light. The solubility of PbI₂ needs to be carefully considered during the preparation of these detectors to ensure the stability and longevity of the material.
3. Environmental Chemistry:
Lead iodide's solubility is relevant in assessing lead contamination in the environment. Knowing its Ksp helps predict the extent of lead leaching into groundwater or soil, aiding in risk assessment and remediation strategies.
4. Analytical Chemistry:
The solubility product is a key parameter in analytical chemistry, especially in gravimetric analysis. Understanding the Ksp of PbI₂ allows chemists to predict the completeness of precipitation and ensure accurate results.
Beyond the Basics: Advanced Concepts
The simple Ksp expression presented above holds true under ideal conditions where ion interactions are negligible. However, in real-world scenarios, especially at higher concentrations, ion activity coefficients should be considered. Activity accounts for the deviation from ideal behavior due to interionic attractions. The modified expression incorporates activity coefficients (γ):
Ksp = (γPb²⁺[Pb²⁺])(γI⁻[I⁻])²
Determining the activity coefficients requires more complex calculations and often relies on experimental data or theoretical models.
Furthermore, the solubility of lead iodide can be affected by the presence of other ions in solution that may form complexes with either Pb²⁺ or I⁻. Accounting for these interactions requires advanced chemical equilibrium models that incorporate multiple equilibria simultaneously.
Conclusion
The solubility product equation for lead iodide, Ksp = [Pb²⁺][I⁻]², is a fundamental concept in chemistry with far-reaching implications. Understanding how to calculate Ksp, the factors affecting solubility, and its application across diverse fields like solar cell technology and environmental science is crucial for researchers, engineers, and scientists. While the basic equation provides a useful framework, it’s essential to recognize the limitations and consider more complex models when dealing with non-ideal conditions. The ability to accurately predict and manipulate the solubility of lead iodide enables advancements in various scientific and technological applications.
Latest Posts
Latest Posts
-
Compute The Number Of Days Sales In Raw Materials Inventory
Apr 01, 2025
-
The Viability Of Suppliers Is Especially Important For Suppliers Who
Apr 01, 2025
-
In General Higher Confidence Levels Provide
Apr 01, 2025
-
Each Doghouse Is A Wooden Structure
Apr 01, 2025
-
What Is The Function Of Structure E
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Write The Equation For The Solubility Product For Lead Iodide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
