Which Statement Describes How A Basic Coffee Cup Calorimeter Works
Holbox
Mar 27, 2025 · 6 min read
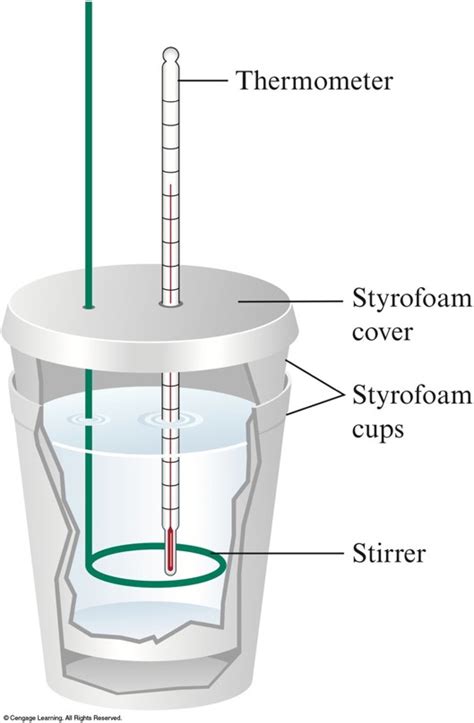
Table of Contents
- Which Statement Describes How A Basic Coffee Cup Calorimeter Works
- Table of Contents
- Which Statement Describes How a Basic Coffee Cup Calorimeter Works? A Deep Dive into Calorimetry
- Understanding the Fundamentals: What is a Coffee Cup Calorimeter?
- The Simplicity and Elegance of the Design:
- How a Coffee Cup Calorimeter Works: A Step-by-Step Explanation
- Exothermic vs. Endothermic Reactions in a Coffee Cup Calorimeter
- Limitations of the Coffee Cup Calorimeter
- Applications of the Coffee Cup Calorimeter
- Improving Accuracy: Advanced Techniques and Considerations
- Conclusion: A Simple Tool with Powerful Applications
- Latest Posts
- Latest Posts
- Related Post
Which Statement Describes How a Basic Coffee Cup Calorimeter Works? A Deep Dive into Calorimetry
Calorimetry, the science of measuring heat, plays a crucial role in various fields, from chemistry and physics to environmental science and engineering. A fundamental tool in calorimetry is the coffee cup calorimeter, a simple yet effective device used to determine the heat transferred during a chemical or physical process. But how does it actually work? This article delves deep into the mechanics of a basic coffee cup calorimeter, explaining its principles, limitations, and applications.
Understanding the Fundamentals: What is a Coffee Cup Calorimeter?
A coffee cup calorimeter, also known as a constant-pressure calorimeter, is a simple apparatus typically constructed from two nested Styrofoam cups, a lid, and a thermometer. The Styrofoam provides excellent insulation, minimizing heat exchange with the surroundings. This setup allows for relatively accurate measurements of heat changes occurring within the inner cup, where the reaction takes place. The key principle underlying its operation is the conservation of energy: the heat lost by the system (the reacting substances) equals the heat gained by the surroundings (the water in the calorimeter).
The Simplicity and Elegance of the Design:
The beauty of the coffee cup calorimeter lies in its simplicity. Its low cost and readily available materials make it an accessible tool for educational and basic research purposes. The ease of use and minimal setup time further contribute to its popularity. However, this simplicity comes with certain limitations, which we'll discuss later.
How a Coffee Cup Calorimeter Works: A Step-by-Step Explanation
The operation of a coffee cup calorimeter hinges on the accurate measurement of temperature changes. The process typically involves these steps:
-
Calibration (Optional but Recommended): While not always performed, calibrating the calorimeter can significantly improve the accuracy of measurements. This involves measuring the heat capacity of the calorimeter itself. A known amount of hot water is added to a known amount of cold water inside the calorimeter. The temperature change is measured, and the calorimeter's heat capacity (C<sub>cal</sub>) is calculated using the equation:
q<sub>cal</sub> = C<sub>cal</sub>ΔT<sub>cal</sub>where:
q<sub>cal</sub>is the heat absorbed by the calorimeterC<sub>cal</sub>is the heat capacity of the calorimeterΔT<sub>cal</sub>is the change in temperature of the calorimeter
-
Preparation: A known mass of reactants is added to a specific volume of water within the inner cup. The initial temperature (T<sub>initial</sub>) is recorded. It’s crucial to ensure the reactants are thoroughly mixed to ensure uniform heat distribution.
-
Reaction Initiation: The reaction is initiated (e.g., by mixing two solutions). The temperature is continuously monitored using the thermometer.
-
Temperature Measurement: The temperature is recorded at regular intervals until the reaction is complete and the temperature reaches a maximum or minimum (depending on whether the reaction is exothermic or endothermic). The final temperature (T<sub>final</sub>) is noted.
-
Heat Calculation: The heat absorbed or released by the reaction (q<sub>rxn</sub>) can be calculated using the following formula:
q<sub>rxn</sub> = -(q<sub>water</sub> + q<sub>cal</sub>)where:
q<sub>water</sub>is the heat absorbed or released by the water, calculated as:q<sub>water</sub> = m<sub>water</sub>C<sub>water</sub>ΔT<sub>water</sub>(m<sub>water</sub> is the mass of water, C<sub>water</sub> is the specific heat capacity of water, and ΔT<sub>water</sub> is the change in water temperature).
-
Enthalpy Calculation: The enthalpy change (ΔH) of the reaction can be determined by dividing the heat of the reaction (q<sub>rxn</sub>) by the number of moles of the limiting reactant. This gives the enthalpy change per mole of reactant.
Exothermic vs. Endothermic Reactions in a Coffee Cup Calorimeter
The coffee cup calorimeter can be used to study both exothermic and endothermic reactions.
-
Exothermic Reactions: In exothermic reactions, heat is released to the surroundings. This leads to an increase in the temperature of the water in the calorimeter. The calculated q<sub>rxn</sub> will be negative, reflecting the heat loss from the system.
-
Endothermic Reactions: In endothermic reactions, heat is absorbed from the surroundings. This causes a decrease in the temperature of the water in the calorimeter. The calculated q<sub>rxn</sub> will be positive, signifying the heat gain by the system.
Limitations of the Coffee Cup Calorimeter
Despite its simplicity and usefulness, the coffee cup calorimeter has several limitations:
-
Heat Loss to Surroundings: Even with good insulation, some heat exchange with the surroundings is inevitable. This leads to errors in the calculated heat of the reaction.
-
Incomplete Reactions: The calorimeter doesn't ensure complete reaction of the reactants. This can lead to underestimation of the heat of the reaction.
-
Non-constant Pressure: While the pressure remains relatively constant during the reaction, it's not perfectly constant, especially for reactions involving gas evolution.
-
Lack of Stirring: Efficient stirring is often crucial for uniform temperature distribution. Insufficient stirring can result in temperature gradients and inaccuracies.
-
Heat Capacity of the Calorimeter: The heat capacity of the calorimeter itself must be considered for accurate results. Ignoring this can lead to significant errors.
-
Limited Applicability: The coffee cup calorimeter is best suited for reactions involving liquids or solutions. It's less suitable for reactions involving solids or gases unless special modifications are made.
Applications of the Coffee Cup Calorimeter
Despite its limitations, the coffee cup calorimeter has various applications, including:
-
Determining the Enthalpy of Reactions: The primary use is to determine the enthalpy change (ΔH) of chemical reactions, particularly those in solution. This information is crucial in many chemical processes.
-
Measuring the Specific Heat Capacity of Substances: With modifications, it can also be used to measure the specific heat capacity of substances.
-
Studying Heat Transfer: The calorimeter can be employed to study heat transfer processes in a variety of systems.
-
Educational Purposes: Its simplicity makes it an ideal tool for demonstrating the principles of calorimetry in educational settings.
Improving Accuracy: Advanced Techniques and Considerations
While the basic coffee cup calorimeter has limitations, its accuracy can be improved by implementing some advanced techniques:
-
Improved Insulation: Using better insulation materials (e.g., more layers of Styrofoam or vacuum insulation) can reduce heat loss.
-
Accurate Temperature Measurement: Using a more precise thermometer (e.g., a digital thermometer with a high resolution) can improve the accuracy of temperature measurements.
-
Efficient Stirring: Employing a magnetic stirrer with a stir bar ensures uniform mixing and temperature distribution.
-
Calorimeter Calibration: Always calibrate the calorimeter to account for its heat capacity.
-
Control Experiments: Performing control experiments to assess the heat loss to the surroundings is important.
-
Statistical Analysis: Analyzing multiple trials and using statistical methods can improve the reliability of results.
Conclusion: A Simple Tool with Powerful Applications
The coffee cup calorimeter, despite its apparent simplicity, is a powerful tool for understanding the principles of calorimetry and measuring the heat transfer associated with chemical and physical processes. Although its limitations must be acknowledged, its ease of use and relatively low cost make it invaluable for educational purposes and basic research. By understanding its principles and limitations, and by employing appropriate techniques, the coffee cup calorimeter can provide reliable and insightful data on the energetics of various processes. With careful experimentation and consideration of error sources, this inexpensive apparatus can yield meaningful and valuable results.
Latest Posts
Latest Posts
-
What Can Be Done To Prevent A Wandering Baseline
Mar 31, 2025
-
Content Marketing Differs From Advertising In That
Mar 31, 2025
-
Human Anatomy And Physiology By Marieb
Mar 31, 2025
-
Meeting The Ethical Challenges Of Leadership
Mar 31, 2025
-
Juanjo Y Manuel No Encuentran El Puesto De Gafas
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Which Statement Describes How A Basic Coffee Cup Calorimeter Works . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
