Which Statement Best Describes The Atoms In A Solid
Holbox
Mar 17, 2025 · 5 min read
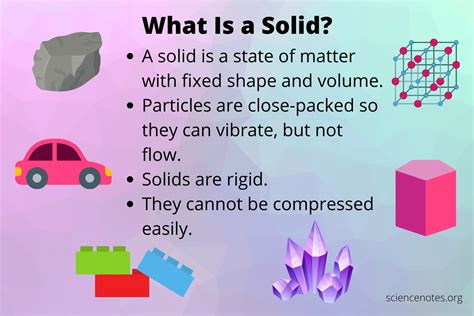
Table of Contents
Which Statement Best Describes the Atoms in a Solid? A Deep Dive into Atomic Structure and Intermolecular Forces
Understanding the behavior of matter hinges on comprehending the nature of its constituent particles. When it comes to solids, the arrangement and interaction of atoms are crucial in determining their properties. This article delves into the atomic structure of solids, exploring various models and statements to definitively answer the question: which statement best describes the atoms in a solid? We'll dissect different descriptions, examining their strengths and limitations in light of the diverse range of solid materials.
Understanding the Nature of Solids: A Macroscopic Perspective
Before diving into the microscopic world of atoms, let's establish a baseline understanding of solids from a macroscopic viewpoint. Solids are characterized by their definite shape and volume. Unlike liquids and gases, they resist changes in both. This rigidity stems from the strong interactions between the constituent particles. We can further classify solids based on their macroscopic properties:
- Crystalline solids: Exhibit a highly ordered, repeating arrangement of atoms, ions, or molecules. This regular structure leads to anisotropy – properties that vary depending on direction. Examples include diamonds, salt crystals, and quartz.
- Amorphous solids: Lack a long-range ordered structure. Atoms are arranged randomly, resembling a frozen liquid. Examples include glass, rubber, and many plastics.
Microscopic Models: Describing Atomic Arrangement in Solids
To understand the atomic-level behavior, we need to move beyond macroscopic observations and examine the various models describing atomic arrangement:
1. The Close-Packed Model: A Simplified View
A simplified model depicts atoms in a solid as hard spheres packed together as tightly as possible. This model effectively explains some properties of solids, particularly the density. However, it's an oversimplification that fails to account for the nuances of chemical bonding and interactions between atoms. While it provides a visual understanding of how atoms might fill space in a solid, it ignores the crucial role of interatomic forces in determining structure and properties.
Limitations: Doesn't account for different types of bonding (ionic, covalent, metallic), crystal structures (cubic, hexagonal, etc.), or the presence of defects within the solid.
2. The Lattice Model: Introducing Order and Regularity
This model introduces the concept of a crystal lattice, a three-dimensional array of points representing the equilibrium positions of atoms, ions, or molecules. The lattice is defined by its unit cell, the smallest repeating unit of the structure. This model is far more sophisticated than the close-packed model and accurately describes the ordered arrangement in crystalline solids. The type of lattice and the atoms within it determine the macroscopic properties of the solid.
Strengths: Explains the ordered structure of crystalline solids, anisotropy, and many physical properties like cleavage planes and diffraction patterns.
Limitations: Doesn't directly describe the nature of bonding forces or the vibrational motion of atoms. It also doesn't easily capture the structure of amorphous solids.
3. The Interatomic Force Model: Bonding and Interactions
This model focuses on the forces of attraction and repulsion between atoms. The atoms in a solid are neither static nor completely independent entities. They are bound together by various interatomic forces, including:
- Ionic bonding: Electrostatic attraction between oppositely charged ions. Found in compounds like NaCl (table salt).
- Covalent bonding: Sharing of electrons between atoms. Found in many non-metallic solids like diamond.
- Metallic bonding: Sharing of electrons in a "sea" of delocalized electrons. Found in metals like copper and iron.
- Van der Waals forces: Weak forces of attraction between molecules or atoms. Found in molecular solids like ice.
The balance between attractive and repulsive forces determines the equilibrium distance between atoms and the strength of the solid. The strength of these forces directly influences the melting point, hardness, and other physical properties.
Strengths: Explains the wide range of properties exhibited by different solids, accounting for the differences in bonding types.
Limitations: Doesn't explicitly capture the complex vibrational motion of atoms or the presence of defects in real-world materials.
Which Statement Best Describes the Atoms in a Solid? A Refined Answer
Considering the various models, the statement that best describes atoms in a solid is a nuanced one that combines aspects of several models:
"The atoms in a solid are arranged in a relatively fixed and ordered or disordered (depending on whether the solid is crystalline or amorphous) structure, held together by strong interatomic forces that determine its properties. The atoms are not static but vibrate around their equilibrium positions, the extent of which depends on temperature."
This statement encompasses several key features:
- Arrangement: Accounts for both the ordered (crystalline) and disordered (amorphous) nature of solids.
- Interatomic forces: Highlights the crucial role of bonding in determining the macroscopic properties.
- Atomic motion: Acknowledges that atoms are not stationary but vibrate, influencing thermal expansion and other phenomena.
Delving Deeper: Factors Affecting Atomic Arrangement
Several factors influence the precise arrangement of atoms in a solid:
- Temperature: Higher temperatures increase atomic vibrations, potentially leading to changes in the structure or even melting.
- Pressure: High pressures can compress the solid, altering interatomic distances and possibly inducing phase transitions.
- Impurities: The presence of foreign atoms (dopants) can significantly affect the properties and structure of a solid.
- Crystal defects: Imperfections in the crystal lattice, such as vacancies and dislocations, can influence mechanical strength and electrical conductivity.
Conclusion: A Holistic Understanding
The behavior of atoms in a solid is a complex interplay of atomic arrangement, interatomic forces, and external factors. While simplified models like the close-packed model provide a basic understanding, a more complete description requires incorporating the concepts of crystal lattices, interatomic forces, and atomic vibrations. Therefore, the refined statement incorporating these elements most accurately captures the dynamic and multifaceted nature of atoms within a solid material. Further investigation into specific solid types and their unique bonding characteristics will provide a more complete picture, but this comprehensive overview provides a solid foundation for understanding this fundamental aspect of material science. Understanding these underlying principles is crucial not only for basic scientific comprehension but also for the design and development of new materials with tailored properties.
Latest Posts
Latest Posts
-
Report For Experiment 9 Properties Of Solutions Answers
Mar 17, 2025
-
Write A Tragic Six Line Poem About Music
Mar 17, 2025
-
Determine The Equation To Be Solved After Removing The Logarithm
Mar 17, 2025
-
Research Shows That People Who Smoke Cigarettes Are More Likely
Mar 17, 2025
-
A Government Created Monopoly Arises When
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Which Statement Best Describes The Atoms In A Solid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
