Which Reactive Species Is Associated With Alzheimer's
Holbox
Mar 22, 2025 · 6 min read
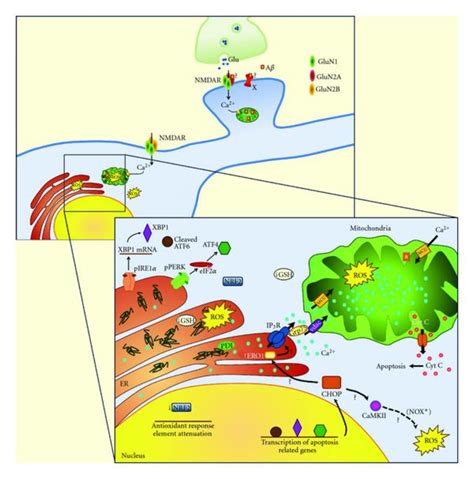
Table of Contents
- Which Reactive Species Is Associated With Alzheimer's
- Table of Contents
- Which Reactive Species is Associated with Alzheimer's?
- The Role of Reactive Oxygen Species (ROS) in Alzheimer's Disease
- 1. Lipid Peroxidation: Damaging Cell Membranes
- 2. Protein Oxidation: Disrupting Cellular Processes
- 3. DNA Damage: Compromising Genetic Integrity
- 4. Mitochondrial Dysfunction: Energy Crisis in Neurons
- Beyond ROS: Other Reactive Species in Alzheimer's
- 1. Reactive Nitrogen Species (RNS): Nitric Oxide and Peroxynitrite
- 2. Advanced Glycation End Products (AGEs): Protein Modification and Inflammation
- 3. Amyloid-β (Aβ) as a Pro-oxidant: Generating ROS and Damaging Neurons
- The Interplay of Reactive Species and Alzheimer's Pathology
- Therapeutic Implications: Targeting Reactive Species
- 1. Antioxidant Therapies: Boosting the Body's Defenses
- 2. Metal Chelation Therapy: Reducing Metal-Catalyzed ROS Formation
- 3. Targeting Mitochondrial Dysfunction: Preserving Energy Production
- 4. Anti-inflammatory Therapies: Reducing Neuroinflammation
- Future Directions and Conclusion
- Latest Posts
- Latest Posts
- Related Post
Which Reactive Species is Associated with Alzheimer's?
Alzheimer's disease (AD), a devastating neurodegenerative disorder, is characterized by progressive cognitive decline and memory loss. While the exact etiology remains elusive, a significant body of research points towards the involvement of reactive oxygen species (ROS) and other reactive species as key players in the disease's pathogenesis. This article delves into the complex relationship between reactive species and Alzheimer's, exploring the different types involved, their mechanisms of action, and the potential therapeutic implications of targeting these species.
The Role of Reactive Oxygen Species (ROS) in Alzheimer's Disease
ROS, including superoxide radicals (O₂⁻), hydroxyl radicals (•OH), and hydrogen peroxide (H₂O₂), are highly reactive molecules generated as byproducts of normal cellular metabolism. However, an imbalance between ROS production and antioxidant defense mechanisms, leading to oxidative stress, is a hallmark of Alzheimer's. This oxidative stress contributes to the disease in several ways:
1. Lipid Peroxidation: Damaging Cell Membranes
ROS readily attack polyunsaturated fatty acids (PUFAs) in cell membranes, initiating a chain reaction called lipid peroxidation. This process leads to membrane damage, disrupting cellular function and integrity. In the context of AD, lipid peroxidation affects neuronal membranes, contributing to synaptic dysfunction and neuronal loss. The resulting malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) are commonly used biomarkers of oxidative stress in AD.
2. Protein Oxidation: Disrupting Cellular Processes
Proteins are also vulnerable targets of ROS. Oxidation of proteins leads to the formation of carbonyl groups, protein aggregation, and loss of function. In AD, this process is implicated in the accumulation of amyloid-β (Aβ) plaques and neurofibrillary tangles (NFTs), two pathological hallmarks of the disease. Specifically, oxidative modifications of tau protein are believed to contribute to NFT formation and neuronal dysfunction. The accumulation of oxidized proteins is a significant indicator of oxidative damage in Alzheimer's.
3. DNA Damage: Compromising Genetic Integrity
ROS can also damage DNA, causing mutations and genomic instability. This damage can affect gene expression and contribute to the progressive neuronal degeneration observed in AD. The accumulation of 8-hydroxy-2'-deoxyguanosine (8-OHdG), a marker of DNA oxidation, is often elevated in the brains of Alzheimer's patients.
4. Mitochondrial Dysfunction: Energy Crisis in Neurons
Mitochondria, the powerhouses of the cell, are particularly susceptible to ROS damage. Mitochondrial dysfunction is a prominent feature of AD, contributing to reduced ATP production and increased ROS generation, creating a vicious cycle. This mitochondrial oxidative stress further exacerbates neuronal damage and contributes to disease progression.
Beyond ROS: Other Reactive Species in Alzheimer's
While ROS are central to the oxidative stress hypothesis of Alzheimer's, other reactive species are also implicated:
1. Reactive Nitrogen Species (RNS): Nitric Oxide and Peroxynitrite
Reactive nitrogen species (RNS), such as nitric oxide (NO) and peroxynitrite (ONOO⁻), are produced by the enzyme nitric oxide synthase (NOS). While NO has some beneficial roles in neuronal signaling, excessive production can lead to the formation of peroxynitrite, a highly reactive species that can damage lipids, proteins, and DNA. Peroxynitrite is particularly damaging to mitochondria and contributes to mitochondrial dysfunction in AD.
2. Advanced Glycation End Products (AGEs): Protein Modification and Inflammation
Advanced glycation end products (AGEs) are formed through the non-enzymatic glycation of proteins. They accumulate with age and are elevated in AD brains. AGEs contribute to oxidative stress by inducing ROS production and promoting inflammation. Their interaction with the receptor for AGE (RAGE) leads to further inflammation and neuronal damage.
3. Amyloid-β (Aβ) as a Pro-oxidant: Generating ROS and Damaging Neurons
Aβ peptides, the primary component of senile plaques, are not only toxic themselves but also act as pro-oxidants, enhancing ROS production and exacerbating oxidative stress. Aβ can bind to metal ions like copper and iron, catalyzing the formation of highly reactive hydroxyl radicals through the Fenton reaction. This contributes significantly to oxidative damage in AD.
The Interplay of Reactive Species and Alzheimer's Pathology
The reactive species mentioned above don't act in isolation; they interact in complex ways to contribute to AD pathogenesis. For instance, ROS can trigger the production of RNS, and both can contribute to Aβ aggregation and neuroinflammation. This intricate interplay of different reactive species makes understanding and targeting them a challenging yet crucial aspect of Alzheimer's research.
Therapeutic Implications: Targeting Reactive Species
Given the significant role of reactive species in Alzheimer's, strategies aimed at reducing their production or enhancing antioxidant defense mechanisms hold immense therapeutic potential. Several approaches are being explored:
1. Antioxidant Therapies: Boosting the Body's Defenses
Antioxidant therapies aim to increase the body's capacity to neutralize ROS and RNS. This includes the use of vitamin E, vitamin C, coenzyme Q10, and other antioxidants. While some studies have shown modest benefits, the results have been inconsistent, and larger, well-designed clinical trials are needed.
2. Metal Chelation Therapy: Reducing Metal-Catalyzed ROS Formation
Metal ions, such as iron and copper, can catalyze the formation of highly reactive hydroxyl radicals. Metal chelation therapy, using agents that bind to these metal ions and prevent their participation in ROS generation, is another promising approach. However, the challenge lies in finding chelators that are effective and have minimal side effects.
3. Targeting Mitochondrial Dysfunction: Preserving Energy Production
Since mitochondrial dysfunction is central to AD pathogenesis, strategies aimed at protecting mitochondria from oxidative damage and improving their function are being investigated. This may involve targeting mitochondrial ROS production or enhancing mitochondrial biogenesis.
4. Anti-inflammatory Therapies: Reducing Neuroinflammation
Neuroinflammation is closely linked to oxidative stress and contributes to neuronal damage in AD. Anti-inflammatory therapies, aimed at reducing the inflammatory response in the brain, may have a beneficial effect in slowing disease progression.
Future Directions and Conclusion
While considerable progress has been made in understanding the role of reactive species in Alzheimer's, much remains to be uncovered. Future research should focus on:
- Identifying specific reactive species that are most critical to disease pathogenesis.
- Developing more effective and specific antioxidant therapies.
- Investigating the interplay between different reactive species and their synergistic effects.
- Developing biomarkers for early detection and monitoring of oxidative stress in AD.
- Exploring personalized medicine approaches tailored to individual oxidative stress profiles.
In conclusion, reactive oxygen species, reactive nitrogen species, and other reactive species play a pivotal role in the pathogenesis of Alzheimer's disease. Oxidative stress, resulting from an imbalance between ROS production and antioxidant defense, contributes to neuronal damage, protein aggregation, mitochondrial dysfunction, and ultimately, cognitive decline. Targeting these reactive species through various therapeutic strategies holds promise for developing effective treatments for this devastating disease. Further research is crucial to unravel the intricate complexities of this relationship and translate these findings into clinically effective interventions. The quest for effective treatments for Alzheimer's disease continues, with a significant focus on managing and mitigating the damaging effects of reactive species within the brain.
Latest Posts
Latest Posts
-
Peer Groups Are Important To Adolescents Because
Mar 24, 2025
-
Refers To An Automated Phone Call Used To Contact Thousands
Mar 24, 2025
-
Adrian Is An Engineering Intern And Earns 14 An Hour
Mar 24, 2025
-
Select The Correct Answer From Each Drop Down Menu
Mar 24, 2025
-
Which Statement About The Mixed Standard Scale Is Most Accurate
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Which Reactive Species Is Associated With Alzheimer's . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
