Which Product S Would Form Under The Conditions Given Below
Holbox
Mar 14, 2025 · 5 min read
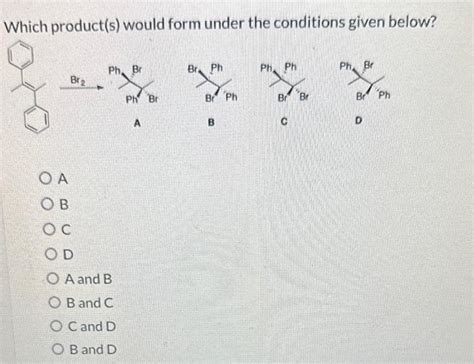
Table of Contents
Predicting Product Formation Under Specific Conditions: A Comprehensive Guide
Understanding which products will form under specific conditions is crucial in various fields, from chemistry and materials science to environmental science and biochemistry. This process involves considering numerous factors, including the reactants involved, temperature, pressure, catalysts, and the presence of solvents. This article delves into the complexities of predicting product formation, exploring different approaches and providing examples to illustrate the underlying principles.
Factors Influencing Product Formation
Predicting the outcome of a chemical reaction requires a systematic analysis of several key factors:
1. Reactants: The Building Blocks
The identity and properties of the reactants are paramount. Different reactants will undergo different reactions, even under identical conditions. For instance, the reaction of sodium metal with water is vastly different from the reaction of iron with water. The nature of the chemical bonds, whether ionic, covalent, or metallic, significantly influences the reaction pathway and the resulting products.
Examples:
-
Reactants: Sodium (Na) and Water (H₂O)
-
Product: Sodium hydroxide (NaOH) and Hydrogen gas (H₂)
-
Reaction: 2Na(s) + 2H₂O(l) → 2NaOH(aq) + H₂(g)
-
Reactants: Iron (Fe) and Water (H₂O)
-
Product: Iron oxide (Fe₂O₃) and Hydrogen gas (H₂) (under high temperature and pressure)
-
Reaction: 4Fe(s) + 3O₂(g) + 6H₂O(l) → 4Fe(OH)₃(s)
The quantity of reactants (stoichiometry) also plays a crucial role. If one reactant is present in excess, it can influence the extent of the reaction and potentially lead to the formation of different products. Limiting reactants dictate the maximum amount of product that can be formed.
2. Temperature: The Energy Driver
Temperature significantly impacts reaction rates and can even determine which products are favored. Increasing temperature generally increases the kinetic energy of molecules, leading to more frequent and energetic collisions, thus increasing the rate of reaction. However, it can also favor different reaction pathways, leading to the formation of different products.
Examples:
- Low Temperature: Some reactions might be too slow to proceed at low temperatures, leading to no significant product formation.
- High Temperature: High temperatures can promote decomposition reactions, leading to different products than those formed at lower temperatures. For example, heating carbonates often leads to the formation of metal oxides and carbon dioxide gas.
3. Pressure: The Compressing Force
Pressure primarily affects reactions involving gases. Increased pressure can shift the equilibrium of reversible reactions towards the side with fewer gas molecules, influencing the product distribution. High pressure can also favor reactions that lead to more compact products.
Examples:
- Haber-Bosch process: The synthesis of ammonia (NH₃) from nitrogen (N₂) and hydrogen (H₂) is highly pressure-dependent. High pressures favor ammonia formation because it has a smaller volume than the reactants.
4. Catalysts: The Reaction Accelerators
Catalysts accelerate reaction rates by providing an alternative reaction pathway with lower activation energy. They do not get consumed in the reaction but can significantly influence the selectivity of a reaction, leading to the preferential formation of certain products.
Examples:
- Enzymes: Biological catalysts that control numerous biochemical reactions, leading to the formation of specific products within living organisms.
5. Solvents: The Reaction Medium
The solvent can significantly influence the reaction rate and product formation. It can affect the solubility of reactants, stabilize intermediates, and even participate directly in the reaction mechanism. The polarity of the solvent is particularly important, with polar solvents favoring polar reactions and non-polar solvents favoring non-polar reactions.
Examples:
- Grignard reactions: These reactions are typically carried out in anhydrous ether solvents because the Grignard reagent is highly reactive with water.
Predicting Product Formation: Approaches and Techniques
Several approaches can be used to predict product formation:
1. Thermodynamic Considerations
Thermodynamics helps determine the feasibility of a reaction and the relative stability of products. The Gibbs free energy (ΔG) change predicts whether a reaction will proceed spontaneously. A negative ΔG indicates a spontaneous reaction, while a positive ΔG indicates a non-spontaneous reaction. However, thermodynamics does not provide information about the reaction rate.
2. Kinetic Considerations
Kinetics studies the rate of reaction and the reaction mechanism. It focuses on the activation energy, which is the energy barrier that must be overcome for a reaction to occur. Lower activation energies lead to faster reactions. Kinetic studies can reveal the reaction intermediates and the rate-limiting step, helping to predict the products formed.
3. Equilibrium Considerations
Many reactions are reversible, reaching a state of equilibrium where the rates of the forward and reverse reactions are equal. The equilibrium constant (K) indicates the relative amounts of reactants and products at equilibrium. Le Chatelier's principle states that changes in conditions (temperature, pressure, concentration) will shift the equilibrium to counteract the change.
4. Computational Chemistry
Computational chemistry uses sophisticated computer programs to model chemical systems and predict reaction outcomes. These methods can simulate reactions, calculate energy changes, and provide insights into reaction mechanisms. However, computational methods require significant computing power and expertise.
5. Experimental Approaches
Experimental approaches involve conducting the reaction under controlled conditions and analyzing the products formed using various analytical techniques (e.g., chromatography, spectroscopy). This approach is essential to validate predictions made by thermodynamic, kinetic, and computational methods.
Examples of Product Prediction in Different Contexts
Let’s consider some specific examples illustrating how the above factors influence product formation:
1. Acid-Base Reactions
Predicting the products of acid-base reactions relies on understanding the relative strengths of acids and bases. Stronger acids will react with stronger bases to form a salt and water. The nature of the salt formed depends on the specific acid and base involved.
2. Redox Reactions
Redox reactions involve electron transfer between reactants. Predicting the products requires considering the oxidation states of the elements involved and their tendency to gain or lose electrons. The use of standard reduction potentials can help determine the feasibility and direction of redox reactions.
3. Organic Reactions
Organic reactions often involve complex mechanisms and multiple possible products. Predicting the major product often requires considering steric factors, electronic effects, and reaction conditions. For example, the regioselectivity and stereoselectivity of addition reactions are heavily influenced by the nature of the reactants and the reaction conditions.
Conclusion: A Holistic Approach
Predicting product formation is a complex process requiring a holistic approach that considers the interplay of various factors. While thermodynamic, kinetic, and computational methods can provide valuable insights, experimental validation remains crucial. A thorough understanding of reaction mechanisms, coupled with the ability to manipulate reaction conditions, is vital for controlling product formation and achieving desired outcomes in various applications. Further research and development in computational chemistry and experimental techniques will continue to enhance our ability to accurately predict and control chemical reactions.
Latest Posts
Latest Posts
-
A Constraint In A Decision Is Blank
Mar 15, 2025
-
The Goal Of Is To Share Resources Especilaly
Mar 15, 2025
-
How Are Diploid Cells Homologous Chromosomes And Alleles Related
Mar 15, 2025
-
Early Americans Preference For Limited Government Was Strengthened By
Mar 15, 2025
-
Are Those Who Are Resonsible For Managing Large
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Which Product S Would Form Under The Conditions Given Below . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
