Which One Of These Is An Amino Group
Holbox
Mar 26, 2025 · 7 min read
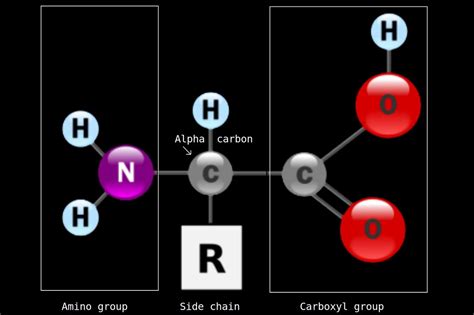
Table of Contents
- Which One Of These Is An Amino Group
- Table of Contents
- Which One of These is an Amino Group? Understanding Amino Acids and Their Building Blocks
- What is an Amino Group?
- Identifying an Amino Group in Chemical Structures
- The Importance of Amino Groups in Amino Acids
- 1. Acid-Base Properties:
- 2. Peptide Bond Formation:
- 3. Protein Structure and Function:
- Differentiating Amino Groups from Similar Functional Groups
- Amide Group (-CONH₂)
- Imine Group (-C=N-)
- Nitro Group (-NO₂)
- Nitrile Group (-CN)
- Amino Groups and Beyond: Exploring the World of Organic Chemistry
- Practical Applications and Further Study
- Latest Posts
- Latest Posts
- Related Post
Which One of These is an Amino Group? Understanding Amino Acids and Their Building Blocks
Amino acids. The very words conjure images of complex biochemistry, protein synthesis, and the building blocks of life itself. But at its core, understanding amino acids starts with grasping their fundamental components, and arguably the most important of these is the amino group. This article will delve deep into the world of amino groups, explaining what they are, their importance in amino acids, and how to identify them within a chemical structure. We'll explore various examples and dispel common misconceptions, providing you with a comprehensive understanding of this crucial functional group.
What is an Amino Group?
An amino group is a functional group characterized by a nitrogen atom bonded to two hydrogen atoms (-NH₂). It's a crucial component of many organic molecules, most notably amino acids, which are the fundamental building blocks of proteins. The nitrogen atom in the amino group possesses a lone pair of electrons, making it a base capable of accepting a proton (H⁺). This property is critical to the chemical reactivity and biological functions of molecules containing amino groups. The ability to act as a base allows amino groups to participate in various chemical reactions, including:
- Acid-base reactions: The amino group readily accepts a proton, forming an ammonium ion (-NH₃⁺). This property significantly influences the overall charge and properties of the molecule.
- Formation of peptide bonds: The reaction between the carboxyl group (-COOH) of one amino acid and the amino group of another is fundamental to protein synthesis. This reaction creates a peptide bond, linking amino acids together to form polypeptide chains.
- Reactions with other functional groups: Amino groups can participate in a variety of other chemical reactions, such as acylation, alkylation, and diazotization, further expanding their role in biological processes.
The presence and position of the amino group in a molecule significantly influence its chemical properties and biological activity.
Identifying an Amino Group in Chemical Structures
Identifying an amino group in a chemical structure is relatively straightforward. Look for a nitrogen atom (N) bonded to two hydrogen atoms (H). This -NH₂ group represents the amino functional group. It's important to remember that the amino group might be attached to a carbon atom, which is often part of a larger organic molecule. Don't be confused by the presence of other atoms or groups surrounding the nitrogen; the core structure of -NH₂ is what defines an amino group.
Let's look at some examples:
Example 1: Glycine
Glycine is the simplest amino acid. Its structure includes a carboxyl group (-COOH), an amino group (-NH₂), and a hydrogen atom (-H) bonded to the central alpha-carbon. The amino group is clearly identifiable as the -NH₂ attached to the alpha carbon.
Example 2: Alanine
Alanine has a similar structure to glycine, but instead of a hydrogen atom, a methyl group (-CH₃) is attached to the alpha-carbon. Again, the -NH₂ group is easily identifiable as the amino group.
Example 3: More Complex Structures
In more complex molecules, the amino group may be less obvious at first glance. However, by systematically identifying the nitrogen atoms and their bonding partners, you can easily pinpoint the amino groups. It's crucial to carefully examine the bonding structure and not just rely on visual inspection.
Example 4: Amides
It's crucial to differentiate between amino groups and amide groups. While amides contain a nitrogen atom, one of the hydrogen atoms in the amino group is replaced by a carbonyl group (-C=O). Amide groups (-CONH₂) are different functional groups with distinct chemical properties.
The Importance of Amino Groups in Amino Acids
The amino group plays a pivotal role in the properties and functions of amino acids. Let's examine this in detail:
1. Acid-Base Properties:
The amino group's ability to act as a base is critical to the behavior of amino acids in solution. The amino group can accept a proton (H⁺), forming a positively charged ammonium ion (-NH₃⁺). This property contributes to the overall charge of the amino acid and its interactions with other molecules. The pKa of the amino group is typically around 9-10, which means that at physiological pH (around 7.4), the amino group is mostly protonated (-NH₃⁺).
2. Peptide Bond Formation:
As mentioned earlier, the reaction between the carboxyl group of one amino acid and the amino group of another is essential for forming peptide bonds. This reaction is a condensation reaction, releasing a water molecule and forming a covalent bond between the two amino acids. This process is the foundation of protein synthesis, where amino acids are linked together to create long polypeptide chains, which fold and assemble into functional proteins.
3. Protein Structure and Function:
The amino group's influence extends beyond the formation of peptide bonds. The charge and interactions of the amino groups within a polypeptide chain contribute to the protein's overall three-dimensional structure. The interaction between amino groups and other functional groups, such as carboxyl groups or side chains, helps to stabilize the protein's conformation and influence its function. This includes the formation of hydrogen bonds, salt bridges, and other non-covalent interactions.
Differentiating Amino Groups from Similar Functional Groups
It's important to distinguish amino groups from other nitrogen-containing functional groups, which might appear similar at first glance. Here's a comparison:
Amide Group (-CONH₂)
As discussed, amides have a carbonyl group (-C=O) attached to the nitrogen. This difference greatly affects the reactivity and properties of the group. Amides are generally less basic than amines.
Imine Group (-C=N-)
Imines have a carbon-nitrogen double bond. This double bond significantly alters the reactivity and properties compared to the amino group's single bond.
Nitro Group (-NO₂)
Nitro groups contain a nitrogen atom double-bonded to two oxygen atoms. They are electron-withdrawing groups and significantly different from amino groups in terms of reactivity and properties.
Nitrile Group (-CN)
Nitriles have a triple bond between carbon and nitrogen. This group is highly polar and significantly differs chemically from the amino group.
Amino Groups and Beyond: Exploring the World of Organic Chemistry
Understanding the amino group is a key stepping stone in comprehending the complexities of organic chemistry and biochemistry. This fundamental functional group plays a critical role in a vast array of biological molecules and chemical processes. By understanding its structure, properties, and interactions, we gain insights into the intricate mechanisms of life itself. This knowledge extends far beyond just identifying the -NH₂ group; it’s about appreciating its contribution to the larger picture of molecular structure and function. From the simple amino acids that build proteins to the more complex molecules that participate in metabolic pathways, the amino group's influence is undeniable. Furthermore, understanding this basic functional group helps build a solid foundation for exploring more advanced concepts in organic and bio-organic chemistry. The journey of discovery in chemistry is continuous, and understanding the amino group is a crucial step in that journey.
Practical Applications and Further Study
The information presented here is just the beginning. To further expand your understanding, consider exploring these avenues:
- Advanced Organic Chemistry Textbooks: These textbooks provide more detailed information on the reactivity and mechanisms of amino groups and their participation in various chemical reactions.
- Biochemistry Textbooks: These delve into the role of amino acids and proteins in biological systems, detailing how amino groups contribute to protein structure, function, and interactions.
- Online Resources: Numerous websites and educational platforms offer interactive tutorials and simulations for visualizing and understanding molecular structures and reactions.
By continuing your exploration of amino groups and their related concepts, you’ll gain a deeper appreciation of the intricacies of the molecular world and the crucial role they play in the biological processes of life. Remember, continued learning and exploration are key to unlocking the wonders of chemistry.
Latest Posts
Latest Posts
-
Per Company Policy Tools With A Purchase
Mar 31, 2025
-
When Should You Introduce Distractor Trials
Mar 31, 2025
-
Which Statement About The Need For Faster Speed To Market Is True
Mar 31, 2025
-
Identify The Four Postulates Of Natural Selection
Mar 31, 2025
-
Select The Action For Which The Featured Muscle Is Responsible
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Which One Of These Is An Amino Group . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
