Which Of The Following Studies Would Need Irb Approval
Holbox
Mar 27, 2025 · 6 min read
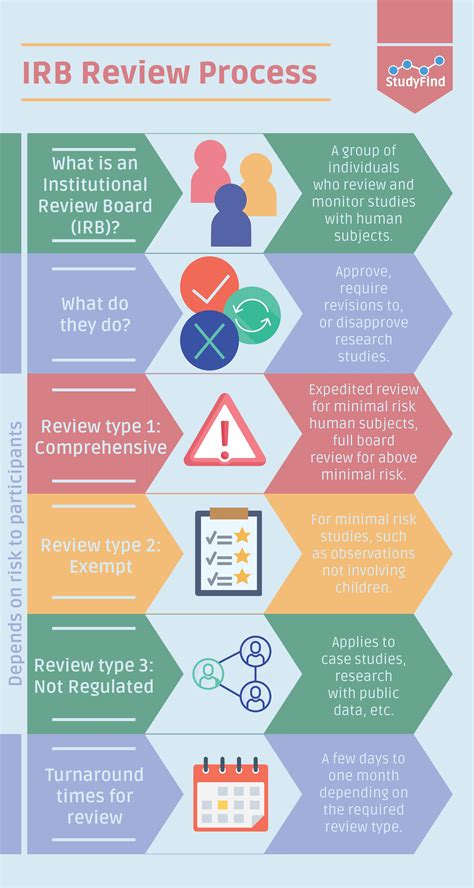
Table of Contents
- Which Of The Following Studies Would Need Irb Approval
- Table of Contents
- Which Studies Need IRB Approval? A Comprehensive Guide
- Understanding IRB and its Purpose
- Key Criteria for IRB Approval
- 1. Involvement of Human Subjects
- 2. Research Involving Intervention or Interaction
- 3. Potential Risks to Participants
- 4. Type of Research Conducted
- Studies That Likely DO NOT Need IRB Approval
- The Importance of Informed Consent
- Conclusion: Prioritize Ethical Conduct
- Latest Posts
- Latest Posts
- Related Post
Which Studies Need IRB Approval? A Comprehensive Guide
Navigating the world of research ethics can be complex, especially when determining whether a study requires Institutional Review Board (IRB) approval. This comprehensive guide will clarify the criteria used to determine which studies necessitate IRB review, providing you with a clear understanding of the regulations and best practices. We'll explore various scenarios and highlight key considerations to ensure ethical and compliant research practices.
Understanding IRB and its Purpose
Before diving into specifics, let's establish a clear understanding of what an IRB is and why it's crucial. An Institutional Review Board (IRB) is an independent committee tasked with reviewing research proposals involving human subjects to ensure the protection of their rights and welfare. Their primary goal is to minimize risks and maximize benefits for participants. This protection is vital and forms the bedrock of ethical research.
IRB approval is not merely a bureaucratic hurdle; it's a critical safeguard against potential harm to participants. By scrutinizing research proposals, IRBs ensure researchers adhere to ethical principles and legal requirements, ultimately promoting trust and integrity within the research community.
Key Criteria for IRB Approval
Several key factors determine whether a study requires IRB approval. These factors are intertwined and often necessitate a holistic evaluation of the research project. Let's dissect these crucial elements:
1. Involvement of Human Subjects
This is the most fundamental criterion. If your research involves human subjects, it almost certainly requires IRB review. But what constitutes a "human subject"? The definition is broader than you might initially think. It includes:
- Individuals: This is straightforward – any research involving individual people.
- Data from Individuals: Even if you don't directly interact with individuals, if your research uses identifiable private information (e.g., medical records, survey responses with identifiers), it involves human subjects.
- Living Individuals: Research involving deceased individuals typically does not require IRB review, unless their information can be linked back to living relatives.
- Embryos, Fetuses, and other biological materials: Research involving these requires careful consideration and often necessitates IRB review.
Example Scenarios:
- Scenario A: A survey about coffee preferences: If the survey includes identifiers linking responses to specific individuals, IRB approval is likely needed. If anonymized, IRB approval might not be required, depending on institutional policy.
- Scenario B: Analyzing publicly available data sets: If the data is anonymized and doesn't contain any personally identifiable information (PII), IRB review may not be necessary. However, if there's a risk of re-identification, IRB approval is highly recommended.
- Scenario C: Observational study in a public park: If the observation is passive and doesn't involve interaction or data collection from individuals, IRB review might be unnecessary. However, if researchers collect identifiable data, such as photos or notes with descriptions, IRB review is typically required.
2. Research Involving Intervention or Interaction
Not all studies involving human subjects require IRB approval. For example, analyzing publicly available data without intervention or interaction might not. However, if your research involves any level of intervention or interaction with participants, IRB review is virtually always mandatory.
- Intervention: This includes any action taken by the researcher that alters the participants' experience or environment. Examples include administering a treatment, providing an intervention, or manipulating a variable.
- Interaction: This encompasses any communication or interaction between the researcher and the participant, such as interviews, surveys, or focus groups.
Example Scenarios:
- Scenario A: A clinical trial testing a new drug: This clearly requires IRB approval due to the significant intervention involved.
- Scenario B: A qualitative study involving interviews: The interaction between the researcher and participants necessitates IRB approval.
- Scenario C: A study using existing datasets without contacting participants: This might not require IRB approval, depending on the nature of the data and the potential for re-identification.
3. Potential Risks to Participants
The potential for harm, both physical and psychological, is a critical factor in determining IRB approval necessity. Even minimal risk studies require review. IRBs assess the risks involved, considering:
- Physical risks: This includes the potential for physical injury, illness, or death.
- Psychological risks: This includes emotional distress, anxiety, or other psychological harm.
- Social risks: This includes the potential for stigma, discrimination, or other social consequences.
- Privacy risks: This includes the potential for breach of confidentiality or unauthorized disclosure of personal information.
Example Scenarios:
- Scenario A: A study involving blood draws: The physical risks associated with blood draws require thorough consideration and IRB review.
- Scenario B: A study exploring sensitive topics like trauma or abuse: The psychological risks require careful attention and necessitate IRB approval.
- Scenario C: A study using de-identified data: The risk of re-identification should be carefully assessed and mitigated, even with de-identified data.
4. Type of Research Conducted
The type of research also influences the need for IRB approval. Certain types of research are inherently more likely to require review due to the nature of the methods or subject matter:
- Qualitative research: Studies involving interviews, focus groups, or observations can pose ethical challenges related to privacy, confidentiality, and potential psychological distress.
- Quantitative research: Even quantitative studies, especially those involving interventions or surveys, may require IRB review.
- Experimental research: Research involving manipulation of variables or interventions often necessitates IRB review due to the increased potential for risk.
- Clinical trials: These studies are always subject to stringent IRB review due to the potential for significant risks to participants.
Studies That Likely DO NOT Need IRB Approval
While many studies require IRB approval, some fall outside its purview. However, even for these, consulting with your institution's IRB or ethics committee is always recommended to ensure compliance and best practices. Examples include:
- Research using publicly available data without identifiers: This typically does not require IRB approval, but careful consideration of potential re-identification is crucial.
- Studies that only analyze existing data or documents that are already in the public domain: Provided there is no identification of individuals, IRB approval may not be needed.
- Educational activities and routine classroom instruction: These are usually exempt from IRB review.
- Certain quality improvement projects within a healthcare setting: These often fall under different regulatory bodies than research ethics boards.
The Importance of Informed Consent
Regardless of whether a study requires IRB approval, obtaining informed consent is paramount. Informed consent ensures that participants are fully aware of the study's purpose, procedures, risks, and benefits before participating. It’s a cornerstone of ethical research and protects participants' autonomy.
Informed consent should be:
- Voluntary: Participants must be free to participate or withdraw at any time without penalty.
- Informed: Participants must receive clear and understandable information about the study.
- Competent: Participants must have the capacity to understand the information and make a decision.
Conclusion: Prioritize Ethical Conduct
Determining whether a study requires IRB approval involves a careful consideration of several interconnected factors. The key is to prioritize ethical conduct and protect the rights and welfare of human participants. When in doubt, always consult with your institution's IRB or ethics committee. Failing to obtain necessary IRB approval can have serious legal and ethical repercussions, potentially jeopardizing your research and your institution's reputation. Proactive engagement with ethical guidelines ensures research integrity and fosters trust in the scientific community. Remember that ethical research is not just about complying with regulations; it's about upholding the highest standards of responsibility and respect for human subjects. A thorough understanding of these criteria is crucial for conducting ethical and impactful research.
Latest Posts
Latest Posts
-
The Difference Between A Budget And A Standard Is That
Mar 30, 2025
-
When Testing A 110 Block The Test Adapter Is Placed
Mar 30, 2025
-
Consider The Accompanying Supply And Demand Graph
Mar 30, 2025
-
What Is The Simplified Form Of The Following Expression
Mar 30, 2025
-
Which Of The Following Are Potential Drawbacks Of Ai
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Studies Would Need Irb Approval . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
