Which Choice Best Describes The Purpose Of Most Pharmacogenomic Research
Holbox
Mar 24, 2025 · 6 min read
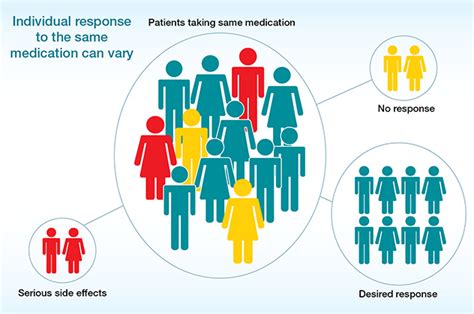
Table of Contents
- Which Choice Best Describes The Purpose Of Most Pharmacogenomic Research
- Table of Contents
- Which Choice Best Describes the Purpose of Most Pharmacogenomic Research?
- Beyond Generic Prescriptions: The Drive for Personalized Medicine
- 1. Optimize Drug Efficacy: The Right Drug, The Right Dose, The Right Patient
- 2. Minimizing Adverse Drug Reactions: Predicting and Preventing Harm
- The Broader Impact: Beyond Individual Patients
- 3. Public Health and Resource Management
- 4. Drug Development and Innovation
- 5. Ethical Considerations and Equity
- Current Applications and Future Directions
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Which Choice Best Describes the Purpose of Most Pharmacogenomic Research?
Pharmacogenomics, the study of how genes affect a person's response to drugs, is rapidly transforming healthcare. Its primary purpose isn't simply to identify genetic variations; it's far more ambitious. The core aim of most pharmacogenomic research is to improve the safety and efficacy of drug therapy by tailoring treatment to an individual's genetic makeup. This encompasses a wide range of activities, from identifying patients most likely to benefit from a specific drug to predicting adverse drug reactions before they occur. This article will delve deep into the multifaceted purposes of pharmacogenomic research, exploring its current applications and future potential.
Beyond Generic Prescriptions: The Drive for Personalized Medicine
The traditional "one-size-fits-all" approach to drug prescription is increasingly recognized as inadequate. Individuals react differently to the same medication due to variations in their genes. These variations influence how drugs are absorbed, metabolized, transported, and ultimately, how they exert their therapeutic effects. Pharmacogenomic research directly tackles this challenge by aiming to:
1. Optimize Drug Efficacy: The Right Drug, The Right Dose, The Right Patient
A major goal of pharmacogenomic research is to identify which patients are most likely to respond positively to a specific drug. This involves:
-
Identifying predictive biomarkers: Researchers are actively searching for genetic markers that correlate with a drug's effectiveness. The presence or absence of specific gene variants can indicate whether a patient is likely to experience a significant benefit or minimal improvement from a given treatment. This precision allows for more effective resource allocation and avoids unnecessary exposure to drugs that are unlikely to work.
-
Determining optimal dosing: Genetic variations can significantly influence how quickly a body metabolizes a drug. Some individuals metabolize drugs faster than others, requiring higher doses to achieve therapeutic levels. Conversely, slow metabolizers might experience increased side effects at standard doses. Pharmacogenomic research aims to establish optimal dosing strategies based on an individual's genotype, minimizing the risk of underdosing (ineffective treatment) or overdosing (toxicity).
-
Developing targeted therapies: By understanding the genetic basis of disease, researchers can develop drugs that specifically target the underlying genetic defect. This approach is particularly promising in areas like cancer treatment, where therapies are being designed to target specific mutations driving tumor growth.
2. Minimizing Adverse Drug Reactions: Predicting and Preventing Harm
Adverse drug reactions (ADRs) are a major concern in healthcare, leading to hospitalizations, increased healthcare costs, and even death. Pharmacogenomic research plays a crucial role in minimizing ADRs by:
-
Identifying susceptibility genes: Some individuals are genetically predisposed to experiencing specific ADRs. Pharmacogenomic research identifies these genes, allowing clinicians to predict and prevent potential problems. For example, certain genetic variations can increase the risk of developing a serious skin reaction to certain antibiotics.
-
Developing safer drugs: By understanding how genetic variations contribute to ADRs, researchers can design drugs with improved safety profiles. This may involve modifying the drug's chemical structure to reduce its interaction with genes that increase the risk of adverse effects.
-
Improving drug monitoring: Pharmacogenomic testing can inform the monitoring of patients receiving medications with a high risk of adverse reactions. This allows for early detection of potential problems and timely intervention.
The Broader Impact: Beyond Individual Patients
The implications of pharmacogenomic research extend far beyond individual patient care. It significantly impacts:
3. Public Health and Resource Management
-
Cost-effectiveness: By improving drug efficacy and reducing ADRs, pharmacogenomics can significantly reduce healthcare costs. Avoiding ineffective treatments and preventing hospitalizations due to ADRs contributes to a more sustainable healthcare system.
-
Enhanced public health initiatives: Pharmacogenomic data can inform public health strategies by identifying populations at increased risk of specific ADRs or those who are less likely to respond to certain medications. This allows for targeted interventions and resource allocation.
4. Drug Development and Innovation
-
Faster drug development: Pharmacogenomic research can accelerate the drug development process by identifying promising drug targets and predicting potential efficacy and safety issues early on. This reduces the time and resources required to bring new drugs to market.
-
More effective drug design: Understanding the genetic basis of drug response allows for the design of more effective and safer medications. This includes creating drugs that specifically target certain genetic variants involved in disease or ADRs.
5. Ethical Considerations and Equity
As pharmacogenomic research advances, ethical considerations and equity become crucial:
-
Access and affordability: Ensuring equitable access to pharmacogenomic testing and personalized treatment is essential to prevent healthcare disparities. Efforts are needed to make these technologies affordable and accessible to all populations.
-
Data privacy and security: The handling and storage of genetic data require robust privacy protections. Strict regulations and ethical guidelines are essential to ensure patient confidentiality and prevent misuse of sensitive information.
-
Informed consent: Patients need to be fully informed about the benefits and risks of pharmacogenomic testing before consenting to participate in research or receive personalized treatment.
Current Applications and Future Directions
Pharmacogenomic testing is already being used clinically for several medications, particularly those with a narrow therapeutic index (the difference between the therapeutic dose and the toxic dose) and those known to have significant inter-individual variability in response. These include:
-
Warfarin (anticoagulant): Genetic testing helps determine the appropriate dose of warfarin to prevent both bleeding and clotting complications.
-
Clopidogrel (antiplatelet): Genetic variations influence the effectiveness of clopidogrel, and testing can identify patients who may require alternative medications.
-
Certain antidepressants and antipsychotics: Pharmacogenomic testing can help predict the response to these medications and potentially avoid the need for trial-and-error approaches.
-
Cancer therapies: Targeted cancer therapies are increasingly guided by pharmacogenomic testing, which identifies specific genetic mutations driving tumor growth.
The future of pharmacogenomic research is exceptionally bright. Advancements in genomic technologies, data analytics, and artificial intelligence will further enhance our understanding of drug response and lead to more precise and personalized medicine. Future research will likely focus on:
-
Integrating pharmacogenomics with other "omics" data: Combining pharmacogenomic data with proteomic, metabolomic, and microbiome data will provide a more comprehensive understanding of drug response.
-
Developing comprehensive pharmacogenomic profiles: The aim is to develop comprehensive profiles that capture an individual's genetic predisposition to drug responses across a wide range of medications.
-
Utilizing artificial intelligence (AI) and machine learning: AI can analyze massive datasets of pharmacogenomic information to identify patterns and predict drug responses more accurately.
Conclusion
In conclusion, the primary purpose of most pharmacogenomic research is to improve the safety and efficacy of drug therapy by tailoring treatment to an individual's genetic makeup. This involves optimizing drug efficacy, minimizing adverse drug reactions, and enhancing public health initiatives. By moving beyond the "one-size-fits-all" approach, pharmacogenomics promises a future of more precise, effective, and safer drug therapy for all. However, realizing this promise requires addressing ethical considerations, ensuring equitable access to testing, and fostering continued research and innovation in this rapidly evolving field. The ultimate goal is not just to treat disease, but to prevent it and to enhance the health and well-being of individuals through a deeply personalized approach to medicine.
Latest Posts
Latest Posts
-
Advertising Goals Listed In An Advertising Plan Must Be
Mar 26, 2025
-
More Rapid Math Tricks And Tips 1st Edition
Mar 26, 2025
-
Match Each Term To The Correct Definition
Mar 26, 2025
-
Identify The Three Major Modes Of Action Of Antiviral Drugs
Mar 26, 2025
-
Label The Structures Of The Urinary Tract In The Figure
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Which Choice Best Describes The Purpose Of Most Pharmacogenomic Research . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
