What Reagents Are Necessary To Carry Out The Conversion Shown
Holbox
Mar 10, 2025 · 5 min read
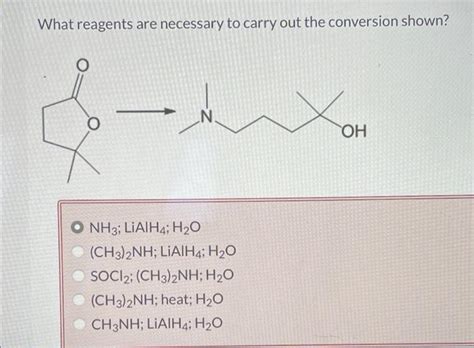
Table of Contents
What Reagents Are Necessary to Carry Out the Conversion Shown? A Comprehensive Guide
This article delves into the crucial aspect of reagent selection in organic chemistry transformations. We'll explore the necessary reagents for a variety of conversions, focusing on the logic behind choosing specific reagents and understanding the reaction mechanisms involved. Successfully executing a chemical conversion hinges on selecting the right reagents; this guide aims to equip you with the knowledge to make informed decisions.
Understanding Reaction Mechanisms: The Foundation of Reagent Selection
Before diving into specific examples, it's crucial to understand that reagent selection is intimately linked to the reaction mechanism. Different reactions proceed through different pathways (SN1, SN2, E1, E2, addition, etc.), and the reagents must be compatible with the desired mechanism. For instance, a strong nucleophile is necessary for an SN2 reaction, while a strong base might be needed for an elimination reaction.
Factors Influencing Reagent Choice:
Several factors must be considered when choosing reagents for a specific conversion:
-
Functionality of the Starting Material: The starting material's functional groups dictate the possible reactions and therefore influence reagent choice. For example, an alcohol can be oxidized, dehydrated, or converted into a halide, each requiring different reagents.
-
Desired Product: The target molecule's structure dictates the necessary transformations and, consequently, the reagents. Synthesizing a specific functional group necessitates reagents capable of introducing that group.
-
Selectivity: Some reagents are highly selective, reacting preferentially with one functional group over another. This selectivity is crucial when multiple reactive groups are present in the starting material.
-
Reaction Conditions: Temperature, solvent, and pressure can significantly impact reagent choice and reaction outcome. Certain reagents may require specific conditions for optimal reactivity.
-
Cost and Availability: While efficacy is paramount, cost and availability also play a role in reagent selection, especially on a large scale.
Examples of Reagent Selection for Different Conversions:
Let's explore several common conversions and the reagents required for each:
1. Alcohol Oxidation to Ketone or Aldehyde:
-
Conversion: Primary alcohol to aldehyde; secondary alcohol to ketone.
-
Reagents: A variety of oxidizing agents can achieve this transformation. Common examples include:
- PCC (Pyridinium chlorochromate): A mild oxidizing agent that selectively oxidizes primary alcohols to aldehydes without further oxidation to carboxylic acids.
- PDC (Pyridinium dichromate): Similar to PCC, but slightly stronger.
- Jones Reagent (Chromic acid): A strong oxidizing agent that oxidizes primary alcohols to carboxylic acids and secondary alcohols to ketones.
- Swern Oxidation: Uses DMSO, oxalyl chloride, and a base like triethylamine. This method is effective for sensitive alcohols.
- Dess-Martin Periodinane (DMP): A mild and selective reagent for oxidizing primary alcohols to aldehydes and secondary alcohols to ketones.
2. Alcohol Conversion to Alkyl Halide:
-
Conversion: Alcohol to alkyl chloride, bromide, or iodide.
-
Reagents: Several reagents can convert alcohols to alkyl halides, the choice depending on the desired halide and the reaction conditions:
- Thionyl Chloride (SOCl2): Converts alcohols to alkyl chlorides. This reagent proceeds via an SN2 mechanism.
- Phosphorus tribromide (PBr3): Converts alcohols to alkyl bromides. This reagent also proceeds via an SN2 mechanism.
- Hydrogen bromide (HBr): Can directly convert alcohols to alkyl bromides. The mechanism is dependent on the alcohol's structure (SN1 or SN2).
- Hydrogen iodide (HI): Converts alcohols to alkyl iodides. Similar mechanism to HBr.
3. Alkene Formation (Dehydration of Alcohols):
-
Conversion: Alcohol to alkene.
-
Reagents: Acid-catalyzed dehydration is a common method for alkene formation from alcohols.
- Strong acids (H2SO4, H3PO4): These acids protonate the alcohol, leading to water elimination and alkene formation. The reaction often follows an E1 mechanism.
4. Grignard Reagent Formation and Reactions:
-
Conversion: Alkyl halide to Grignard reagent (followed by reaction with a carbonyl compound).
-
Reagents:
- Magnesium (Mg): Reacts with alkyl halides in anhydrous ether to form Grignard reagents (RMgX).
- Various carbonyl compounds (aldehydes, ketones, esters, etc.): Grignard reagents act as nucleophiles, attacking the carbonyl carbon to form new carbon-carbon bonds.
5. Esterification:
-
Conversion: Carboxylic acid to ester.
-
Reagents:
- Alcohol: Reacts with the carboxylic acid in the presence of an acid catalyst.
- Acid catalyst (H2SO4): Facilitates the reaction.
6. Amide Formation:
-
Conversion: Carboxylic acid to amide.
-
Reagents:
- Amine: Reacts with the carboxylic acid, often requiring a coupling reagent like DCC (dicyclohexylcarbodiimide) or EDC (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide).
7. Nitrile Reduction to Amine:
-
Conversion: Nitrile to primary amine.
-
Reagents:
- Lithium aluminum hydride (LiAlH4): A strong reducing agent that reduces nitriles to primary amines.
8. Reduction of Ketones and Aldehydes to Alcohols:
-
Conversion: Ketone or aldehyde to alcohol.
-
Reagents: Several reducing agents can accomplish this transformation:
- Sodium borohydride (NaBH4): A mild reducing agent that reduces aldehydes and ketones to primary and secondary alcohols, respectively.
- Lithium aluminum hydride (LiAlH4): A strong reducing agent that can reduce a wider range of functional groups, including esters, acids, and nitriles.
9. Williamson Ether Synthesis:
-
Conversion: Alkyl halide to ether.
-
Reagents:
- Alkoxide ion: Formed by reacting an alcohol with a strong base (e.g., sodium hydride, NaH).
- Alkyl halide: Reacts with the alkoxide ion via an SN2 mechanism.
10. Friedel-Crafts Acylation:
-
Conversion: Benzene to acylbenzene.
-
Reagents:
- Acyl chloride: The electrophile.
- Lewis acid catalyst (AlCl3): Activates the acyl chloride.
This list provides a starting point for understanding reagent selection. Many other conversions and reagents exist, each with its own specific applications and considerations. Always consult reliable sources and consider the reaction mechanism before choosing reagents for a specific transformation.
Advanced Considerations and Future Trends:
Reagent selection is an ongoing area of research, with a constant drive towards greener, more efficient, and selective methods. Several areas warrant consideration:
-
Green Chemistry: The focus on minimizing waste and using environmentally benign reagents is driving the development of new, sustainable methods.
-
Catalysis: The use of catalysts, especially heterogeneous catalysts, is becoming increasingly important in reducing reagent quantities and improving reaction selectivity.
-
Flow Chemistry: Performing reactions in continuous flow reactors offers advantages in terms of control, scalability, and safety.
-
Computational Chemistry: Computational methods are playing an increasingly important role in predicting reaction outcomes and optimizing reagent selection.
By carefully considering the reaction mechanism, the starting material's structure, the desired product, and the available resources, chemists can make informed decisions about reagent selection to achieve efficient and successful chemical transformations. The examples provided in this article represent only a fraction of the vast landscape of organic chemistry reactions; continued learning and exploration are essential for mastering this critical aspect of synthetic chemistry. This comprehensive guide should serve as a valuable resource for students, researchers, and anyone interested in deepening their understanding of reagent selection in organic chemistry.
Latest Posts
Latest Posts
-
A Planned Evacuation Drill Should Occur
Mar 10, 2025
-
Two Fishermen Are In A 4 Meter Boat
Mar 10, 2025
-
Julia Is A Customer Sitting At Your Bar
Mar 10, 2025
-
I Seldom Probe Deeply Into A Subject
Mar 10, 2025
-
What Is The Definition Of Mild And Brief Symptom Exacerbation
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about What Reagents Are Necessary To Carry Out The Conversion Shown . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
