What Is The Relationship Between The Following Compounds
Holbox
Mar 20, 2025 · 5 min read
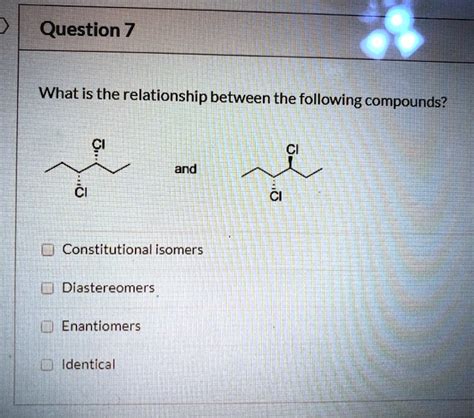
Table of Contents
Delving into the Interrelationships of Chemical Compounds: A Comprehensive Exploration
Understanding the relationships between chemical compounds is fundamental to chemistry. These relationships aren't simply about similarities in structure or properties; they delve into the intricate ways compounds react, transform, and influence one another. This exploration will examine the connections between different chemical compounds, focusing on how they interrelate through various chemical processes and underlying principles. While a specific list of compounds wasn't provided, we'll investigate overarching themes to illustrate these relationships. This will encompass isomerism, functional group relationships, reaction pathways, and the concept of homologous series.
1. Isomerism: The Same Formula, Different Arrangement
Isomers are compounds that share the same molecular formula but possess different structural arrangements. This difference in arrangement leads to variations in their physical and chemical properties. Several types of isomerism exist, including:
-
Structural Isomerism: This involves differences in the connectivity of atoms within the molecule. For example, butane (C₄H₁₀) exists as two structural isomers: n-butane (a straight chain) and isobutane (a branched chain). These isomers differ in their boiling points and reactivity.
-
Stereoisomerism: This arises from differences in the spatial arrangement of atoms or groups within the molecule. Two main subtypes are:
-
Geometric Isomerism (cis-trans isomerism): This occurs in compounds containing double bonds or cyclic structures, where the arrangement of substituents around the double bond or ring differs. For example, cis- and trans-2-butene differ in their dipole moments and physical properties.
-
Optical Isomerism (enantiomerism): This occurs when molecules are non-superimposable mirror images of each other (chiral). These isomers, called enantiomers, often exhibit different interactions with polarized light and biological systems. A classic example is lactic acid, which possesses two enantiomers.
-
2. Functional Group Relationships: The Building Blocks of Reactivity
Functional groups are specific groups of atoms within a molecule that are responsible for its characteristic chemical reactions. The presence of a particular functional group dictates the compound's reactivity and its potential to undergo specific reactions. Understanding functional group relationships allows us to predict the behavior of compounds and design synthesis pathways.
-
Alcohols and Ethers: Both contain an oxygen atom, but alcohols have the general formula R-OH (R represents an alkyl group), while ethers have the formula R-O-R'. This subtle difference drastically affects reactivity. Alcohols are more reactive due to the presence of a polar O-H bond, capable of hydrogen bonding and readily participating in reactions such as oxidation and esterification. Ethers, relatively less reactive, are primarily used as solvents.
-
Aldehydes and Ketones: Both contain a carbonyl group (C=O), but aldehydes have the carbonyl group at the end of a carbon chain (R-CHO), while ketones have it within the chain (R-CO-R'). This difference leads to variations in reactivity. Aldehydes are more readily oxidized than ketones because they possess a hydrogen atom on the carbonyl carbon.
-
Carboxylic Acids and Esters: Carboxylic acids (R-COOH) contain a carboxyl group (-COOH), which is a combination of a carbonyl group and a hydroxyl group. Esters (R-COO-R') are formed by the reaction of a carboxylic acid with an alcohol, replacing the hydroxyl group with an alkoxy group. Carboxylic acids are acidic, while esters are generally neutral.
3. Reaction Pathways: Transformations and Interconversions
The relationships between compounds are also defined by the reaction pathways that interconnect them. These pathways dictate how one compound can be transformed into another, and understanding these pathways is crucial for organic synthesis and chemical analysis.
-
Oxidation-Reduction Reactions: Many compounds can be interconverted through oxidation or reduction reactions. For instance, alcohols can be oxidized to aldehydes or ketones, and aldehydes can be further oxidized to carboxylic acids. Conversely, carboxylic acids can be reduced to aldehydes and alcohols.
-
Addition Reactions: These reactions involve the addition of atoms or groups to a molecule, often across a double or triple bond. For example, alkenes can undergo addition reactions with halogens or hydrogen halides.
-
Substitution Reactions: These involve the replacement of one atom or group with another. Alkyl halides can undergo substitution reactions with nucleophiles, leading to the formation of various functional groups.
-
Elimination Reactions: These involve the removal of atoms or groups from a molecule, often leading to the formation of a double or triple bond. For example, alcohols can undergo dehydration to form alkenes.
4. Homologous Series: Families of Compounds
Homologous series are families of compounds that share the same general formula and functional group, differing only in the length of their carbon chain. Members of a homologous series exhibit similar chemical properties but show gradual changes in physical properties with increasing chain length.
-
Alkanes: These saturated hydrocarbons follow the general formula CₙH₂ₙ₊₂, exhibiting increasing boiling points and melting points with increasing chain length.
-
Alkenes: These unsaturated hydrocarbons with a double bond follow the general formula CₙH₂ₙ, also showing similar trends in physical properties with increasing chain length.
-
Alcohols: These compounds, containing a hydroxyl group (-OH), exhibit increasing boiling points with increasing carbon chain length due to stronger intermolecular forces.
5. Spectroscopic Techniques: Unveiling Compound Relationships
Various spectroscopic techniques play a vital role in determining the structure and identifying the relationships between compounds. Techniques like Nuclear Magnetic Resonance (NMR) spectroscopy, Infrared (IR) spectroscopy, and Mass Spectrometry (MS) provide crucial information about the arrangement of atoms and functional groups present in a molecule, thereby aiding in the determination of isomeric forms and identification of homologous series members.
Conclusion: A Network of Interconnections
The relationships between chemical compounds are multifaceted and intricate. Isomerism highlights how different arrangements of the same atoms can lead to diverse properties. Functional groups serve as the key to predicting reactivity, while reaction pathways demonstrate the dynamic transformations compounds undergo. Homologous series illustrate the systematic variations within families of related molecules. Finally, spectroscopic techniques provide powerful tools for identifying and characterizing these relationships. By understanding these interconnected concepts, we can unlock a deeper appreciation for the complexity and beauty of the chemical world and its underlying principles. This comprehensive understanding forms the basis of advances in fields ranging from drug discovery to materials science and environmental chemistry. Further exploration into specific examples and reaction mechanisms will only enhance this fundamental knowledge.
Latest Posts
Latest Posts
-
Three Infinite Straight Wires Are Fixed In Place And Aligned
Mar 20, 2025
-
Match Each Of The Options Above To The Items Below
Mar 20, 2025
-
Steven Roberts Is A Mental Health Counselor In New Jersey
Mar 20, 2025
-
Match Each Term With The Best Description
Mar 20, 2025
-
A Student Sets Up The Following Equation
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about What Is The Relationship Between The Following Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
