What Is The Missing Reagent In The Reaction Below
Holbox
Mar 25, 2025 · 5 min read
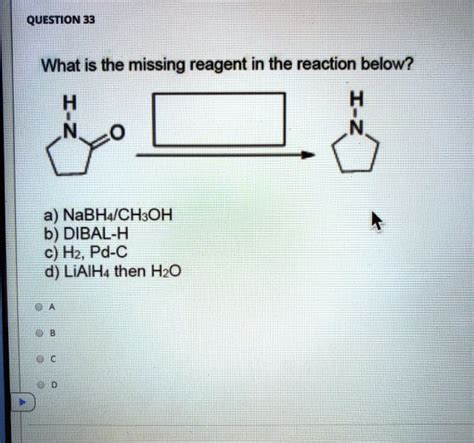
Table of Contents
What's the Missing Reagent in This Reaction? A Deep Dive into Reaction Mechanisms and Synthetic Strategies
Determining the missing reagent in a chemical reaction requires a thorough understanding of reaction mechanisms, functional group transformations, and synthetic strategies. This article will delve into the process of identifying missing reagents, using various examples to illustrate the principles involved. We'll explore common reaction types, analyze reactant structures, and predict potential products to deduce the necessary reagents.
Understanding Reaction Mechanisms: The Key to Identifying Missing Reagents
Before we can even begin to discuss missing reagents, we need to establish a firm grasp of reaction mechanisms. A reaction mechanism describes the step-by-step process by which reactants transform into products. This knowledge is crucial because it reveals the specific chemical transformations required at each step, allowing us to pinpoint the missing reagent.
Common Reaction Types and Their Associated Reagents
Several common reaction types frequently feature in organic chemistry. Understanding these will significantly aid in identifying missing reagents. These include:
1. Oxidation Reactions: These involve the increase in oxidation state of an atom, usually carbon. Common oxidizing agents include:
- Potassium permanganate (KMnO₄): A strong oxidizing agent used to oxidize alcohols to carboxylic acids, alkenes to diols, and aldehydes to carboxylic acids.
- Chromic acid (H₂CrO₄): Another strong oxidizing agent capable of similar transformations as KMnO₄.
- Jones reagent (CrO₃ in H₂SO₄): Specifically used to oxidize primary alcohols to carboxylic acids and secondary alcohols to ketones.
- PCC (pyridinium chlorochromate): A milder oxidizing agent that typically oxidizes primary alcohols to aldehydes and secondary alcohols to ketones.
2. Reduction Reactions: These involve the decrease in oxidation state of an atom. Common reducing agents include:
- Lithium aluminum hydride (LiAlH₄): A powerful reducing agent capable of reducing esters, ketones, aldehydes, and carboxylic acids to alcohols.
- Sodium borohydride (NaBH₄): A milder reducing agent that primarily reduces aldehydes and ketones to alcohols.
- Hydrogen gas (H₂) with a metal catalyst (e.g., Pd/C, Pt): Used for catalytic hydrogenation of alkenes and alkynes to alkanes.
3. Nucleophilic Substitution Reactions: These reactions involve a nucleophile attacking an electrophilic carbon atom, leading to the substitution of a leaving group. Common nucleophiles include:
- Hydroxide ion (OH⁻): A strong nucleophile used in SN1 and SN2 reactions.
- Halide ions (Cl⁻, Br⁻, I⁻): Also used in SN1 and SN2 reactions.
- Alkoxide ions (RO⁻): Used in Williamson ether synthesis.
4. Electrophilic Addition Reactions: These reactions involve the addition of an electrophile to an unsaturated compound, such as an alkene or alkyne. Common electrophiles include:
- Halogens (Cl₂, Br₂, I₂): Add across the double or triple bond of an alkene or alkyne.
- Hydrogen halides (HCl, HBr, HI): Add across the double or triple bond, following Markovnikov's rule.
5. Grignard Reactions: Organomagnesium halides (Grignard reagents) act as nucleophiles, reacting with carbonyl compounds (aldehydes, ketones, esters, carboxylic acids) to form new carbon-carbon bonds.
Analyzing Reactants and Predicting Products: A Step-by-Step Approach
To identify the missing reagent, follow these steps:
-
Identify the functional groups present in the reactants and products: This will help you determine the type of reaction that has occurred.
-
Determine the changes in the structure of the reactants to form the products: This will indicate the specific transformation that has taken place.
-
Consider the reaction mechanisms involved: Understanding the mechanism will reveal the steps required for the transformation, allowing you to identify the missing reagent based on its role in the mechanism.
-
Consult reaction charts and databases: These resources provide a comprehensive overview of common reactions and their corresponding reagents.
Example Scenarios and Solutions:
Let's consider a few examples to illustrate the process:
Example 1: Conversion of an alkene to a vicinal diol.
- Reactant: An alkene
- Product: A vicinal diol (two hydroxyl groups on adjacent carbons)
- Missing Reagent: Osmium tetroxide (OsO₄) followed by a reducing agent like sodium bisulfite (NaHSO₃)
Example 2: Conversion of a ketone to a secondary alcohol.
- Reactant: A ketone
- Product: A secondary alcohol
- Missing Reagent: A reducing agent such as sodium borohydride (NaBH₄) or lithium aluminum hydride (LiAlH₄). LiAlH₄ is a stronger reducing agent and would be more effective, but NaBH₄ might suffice depending on the ketone's structure.
Example 3: Formation of an ether from an alcohol and an alkyl halide.
- Reactants: An alcohol and an alkyl halide
- Product: An ether
- Missing Reagent: A strong base, such as sodium hydride (NaH) or potassium tert-butoxide (t-BuOK). This is a Williamson ether synthesis.
Example 4: Conversion of a carboxylic acid to an ester.
- Reactants: A carboxylic acid and an alcohol
- Product: An ester
- Missing Reagent: An acid catalyst such as sulfuric acid (H₂SO₄) or p-toluenesulfonic acid (TsOH). This is Fischer esterification.
The Importance of Considering Reaction Conditions
Besides identifying the correct reagent, it is critical to consider the reaction conditions. Temperature, solvent, and other factors can significantly influence the outcome of a reaction. The wrong conditions can lead to undesired side reactions or poor yields, even if the correct reagent is used.
Conclusion: A Holistic Approach to Reagent Identification
Identifying the missing reagent in a chemical reaction requires a multifaceted approach. A strong understanding of reaction mechanisms, functional group transformations, and synthetic strategies is paramount. Combining this knowledge with careful analysis of the reactants and products, along with consideration of reaction conditions, will enable you to successfully deduce the necessary reagent(s) for a given transformation. This skill is essential for anyone involved in synthetic organic chemistry, from students to experienced researchers. Consistent practice and careful attention to detail are key to mastering this crucial aspect of organic chemistry. Remember to always consult reliable resources and consider safety precautions when working with chemical reagents.
Latest Posts
Latest Posts
-
Is The Extent To Which People Like Or Dislike Themselves
Mar 28, 2025
-
The Description Is Progressively Throughout The Project
Mar 28, 2025
-
In Which Of The Following Countries Is Cremation Most Popular
Mar 28, 2025
-
All Of The Following Are Functions Of Proteins Except
Mar 28, 2025
-
Purchasing Power Parity Theory Does Not Hold At All Times Because
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Is The Missing Reagent In The Reaction Below . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
