What Is Difference Between Temperature And Heat
Holbox
Mar 09, 2025 · 6 min read
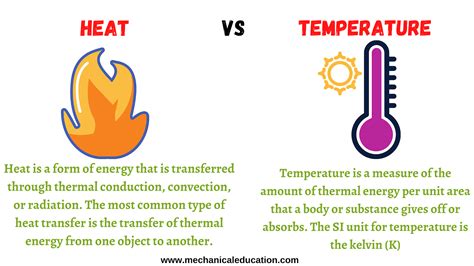
Table of Contents
What's the Difference Between Temperature and Heat? A Deep Dive
Understanding the difference between temperature and heat is crucial for grasping fundamental concepts in physics, chemistry, and even everyday life. While often used interchangeably in casual conversation, these two terms represent distinct physical quantities. This article delves deep into the nuanced differences, exploring their definitions, measurements, and applications, with a focus on providing a comprehensive and easily understandable explanation.
Defining Temperature and Heat: Two Sides of the Same Coin?
Let's start by defining each term precisely:
Temperature: Temperature is a measure of the average kinetic energy of the particles (atoms and molecules) within a substance. It's a measure of how hot or cold something is. Think of it as the average speed at which these particles are moving and vibrating. The higher the average kinetic energy, the higher the temperature. Crucially, temperature is an intensive property, meaning it doesn't depend on the amount of substance present. A cup of boiling water and a pot of boiling water have the same temperature, even though the pot contains more water.
Heat: Heat, on the other hand, is the transfer of thermal energy between objects at different temperatures. It's the flow of energy from a hotter object to a colder one. This transfer continues until thermal equilibrium is reached, meaning both objects reach the same temperature. Heat is an extensive property, meaning it does depend on the amount of substance involved. A larger pot of boiling water contains more heat energy than a smaller cup of boiling water at the same temperature. Heat is often measured in Joules (J) or calories (cal).
Think of it like this: temperature is the measure of how vigorously the particles are moving, while heat is the measure of the total energy being exchanged when two things of differing temperatures come into contact.
Understanding the Microscopic Perspective
To further clarify the difference, let's consider the microscopic perspective:
-
Temperature: At the atomic level, temperature reflects the average speed of the atoms or molecules. In a hot object, particles move rapidly and collide frequently. In a cold object, particles move more slowly and collide less often.
-
Heat: Heat is the transfer of energy that occurs due to the temperature difference between two objects. When a hot object comes into contact with a cold object, the faster-moving particles in the hot object collide with the slower-moving particles in the cold object, transferring kinetic energy. This process continues until the average kinetic energies of the particles in both objects are equal, resulting in thermal equilibrium.
Measurement and Units
Temperature and heat are measured using different scales and units:
Temperature Scales:
-
Celsius (°C): Widely used globally, based on the freezing (0°C) and boiling (100°C) points of water at standard atmospheric pressure.
-
Fahrenheit (°F): Primarily used in the United States, with different freezing (32°F) and boiling (212°F) points for water.
-
Kelvin (K): The absolute temperature scale, where 0 K represents absolute zero – the theoretical point at which all molecular motion ceases. Kelvin is the SI unit for temperature.
Heat Units:
-
Joule (J): The SI unit of energy, including heat energy.
-
Calorie (cal): An older unit of energy, defined as the amount of heat required to raise the temperature of 1 gram of water by 1 degree Celsius. 1 calorie equals 4.184 Joules.
-
British Thermal Unit (BTU): A unit of heat energy commonly used in the United States, defined as the amount of heat required to raise the temperature of 1 pound of water by 1 degree Fahrenheit.
Thermal Equilibrium and Heat Transfer Mechanisms
Heat transfer always occurs from a higher-temperature object to a lower-temperature object until thermal equilibrium is reached. There are three primary mechanisms of heat transfer:
1. Conduction: Heat transfer through direct contact between objects or within a material. For example, touching a hot stove burner transfers heat to your hand. Materials with high thermal conductivity (e.g., metals) transfer heat efficiently, while materials with low thermal conductivity (e.g., wood, plastic) transfer heat slowly.
2. Convection: Heat transfer through the movement of fluids (liquids or gases). For instance, warm air rising from a heater and cooler air sinking creates a convection current.
3. Radiation: Heat transfer through electromagnetic waves. The sun's warmth reaching Earth is an example of radiation. All objects emit thermal radiation, with the amount of radiation increasing with temperature.
Specific Heat Capacity: A Key Concept
Specific heat capacity is a crucial property that relates the amount of heat required to change the temperature of a substance. It's defined as the amount of heat needed to raise the temperature of 1 kilogram of a substance by 1 Kelvin (or 1 degree Celsius). Different substances have different specific heat capacities. Water, for example, has a relatively high specific heat capacity, meaning it requires a significant amount of heat to raise its temperature. This high specific heat capacity is vital for regulating Earth's climate.
Examples in Everyday Life
Understanding the difference between temperature and heat is crucial in many everyday situations:
-
Cooking: A high-temperature oven (temperature) transfers a significant amount of heat (energy) to your food, causing it to cook.
-
Heating a home: A heating system increases the temperature of the air inside your house by transferring heat energy to the air.
-
Cooling a beverage: Adding ice to a warm drink lowers the drink's temperature by transferring heat from the drink to the ice.
Advanced Concepts and Applications
The distinction between temperature and heat extends beyond basic thermodynamics into more complex areas such as:
-
Thermodynamics: The study of heat and its relationship to other forms of energy. Key concepts like entropy, enthalpy, and Gibbs free energy are intricately linked to both temperature and heat transfer.
-
Statistical Mechanics: This field uses statistical methods to link microscopic properties of particles to macroscopic thermodynamic properties like temperature and heat.
-
Material Science: Understanding the thermal properties of materials, including their specific heat capacity and thermal conductivity, is crucial for designing and engineering a wide range of products and systems.
-
Climate Science: Temperature changes, both local and global, driven by heat transfer processes, are central to understanding climate change and its impact on the planet.
Conclusion: A Clear Distinction
While the terms "temperature" and "heat" are often used interchangeably in casual conversation, they represent fundamentally different concepts. Temperature measures the average kinetic energy of particles in a substance, while heat refers to the transfer of thermal energy between objects at different temperatures. Understanding this crucial difference is essential for comprehending various phenomena in physics, chemistry, and engineering, as well as for navigating many aspects of everyday life. By grasping the definitions, measurement methods, and underlying principles, one can appreciate the profound significance of temperature and heat in shaping our world.
Latest Posts
Latest Posts
-
What Percent Is 15 Out Of 35
Mar 09, 2025
-
Plant Adaptations In The Temperate Rainforest
Mar 09, 2025
-
How To Say Canada In French
Mar 09, 2025
-
Lord Of The Flies Chapter Summary
Mar 09, 2025
-
Lord Of The Flies Chapter 9
Mar 09, 2025
Related Post
Thank you for visiting our website which covers about What Is Difference Between Temperature And Heat . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
