What Happens To Cdks In The Absence Of Cyclins
Holbox
Mar 30, 2025 · 6 min read
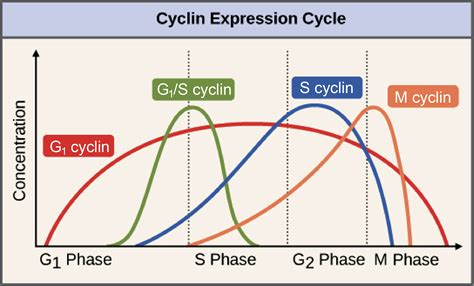
Table of Contents
- What Happens To Cdks In The Absence Of Cyclins
- Table of Contents
- What Happens to CDKs in the Absence of Cyclins?
- The Crucial Role of Cyclins in CDK Activation
- Structural Changes Induced by Cyclin Binding
- Beyond Simple Activation: Cyclin Specificity and CDK Regulation
- Consequences of CDK Activity in the Absence of Cyclins
- Reduced Kinase Activity and Substrate Specificity
- Aberrant Phosphorylation and Cellular Dysfunction
- Impact on Cell Cycle Progression
- Mechanisms Compensating for Cyclin Absence (or attempting to)
- CDK Inhibitors (CKIs) and their Role
- Degradation of CDKs
- Post-translational Modifications
- Clinical Implications and Disease Associations
- Cancer and Dysregulation of Cyclin-CDK Pathways
- Therapeutic Targeting of CDKs
- Conclusion: A Tightly Regulated Dance
- Latest Posts
- Latest Posts
- Related Post
What Happens to CDKs in the Absence of Cyclins?
Cyclin-dependent kinases (CDKs) are a family of serine/threonine-specific protein kinases that regulate a variety of cellular processes, most notably the cell cycle. Their activity is tightly controlled, primarily through the binding of regulatory proteins called cyclins. This article delves into the consequences of CDK activity in the absence of cyclins, exploring the molecular mechanisms, cellular impacts, and broader implications for cell health and disease.
The Crucial Role of Cyclins in CDK Activation
CDKs are inherently inactive without their cyclin partners. While possessing the catalytic machinery for phosphorylation, they lack the structural conformation necessary for efficient substrate binding and enzymatic activity. Cyclin binding induces a conformational change in the CDK, exposing the active site and allowing for substrate interaction. This interaction is more than simply a physical association; it involves crucial structural rearrangements that affect the kinase's catalytic efficiency. Think of it like a key (cyclin) fitting into a lock (CDK) to activate the mechanism.
Structural Changes Induced by Cyclin Binding
The binding of a cyclin to a CDK involves several key structural modifications. Importantly, the cyclin contributes to the formation of the active site cleft, bringing essential amino acid residues into the correct orientation for ATP binding and substrate phosphorylation. Additionally, cyclin binding stabilizes the activation loop, a region that is critical for catalytic activity. In the absence of cyclin, this activation loop adopts a conformation that sterically hinders substrate binding and catalysis.
Beyond Simple Activation: Cyclin Specificity and CDK Regulation
The relationship between CDKs and cyclins is not simply a binary "on/off" switch. Different cyclins bind to specific CDKs, leading to the activation of distinct downstream pathways and the regulation of specific cell cycle phases. This specificity ensures the correct timing and order of cell cycle events. For instance, cyclin D-CDK4/6 complexes are primarily involved in G1 phase progression, whereas cyclin B-CDK1 complexes regulate the G2/M transition. The absence of a specific cyclin, therefore, doesn't just lead to a general lack of CDK activity; it leads to a specific functional deficit in a particular stage of the cell cycle or a cellular process.
Consequences of CDK Activity in the Absence of Cyclins
Although inactive without cyclins, CDKs can exhibit some residual activity in their absence. However, this activity is significantly reduced, and its effects are generally considered aberrant compared to the tightly regulated activity observed in the presence of cyclins.
Reduced Kinase Activity and Substrate Specificity
Without cyclins, CDKs exhibit drastically reduced kinase activity. This is due to the lack of the conformational changes necessary for efficient substrate binding and catalysis. The residual activity that remains might be non-specific, meaning that it might phosphorylate inappropriate substrates. This lack of specificity could lead to the deregulation of cellular processes and contribute to cellular dysfunction. The crucial control mechanism offered by cyclin binding is lost, leading to potentially damaging consequences.
Aberrant Phosphorylation and Cellular Dysfunction
Aberrant phosphorylation due to residual CDK activity can trigger a cascade of downstream effects. For instance, uncontrolled phosphorylation of critical cell cycle regulators could lead to cell cycle arrest, uncontrolled cell growth, or apoptosis (programmed cell death). The cellular outcome significantly depends on which CDK is involved and which substrates are inappropriately phosphorylated.
Impact on Cell Cycle Progression
The absence of cyclins and the subsequent reduction in CDK activity have profound effects on cell cycle progression. For instance, the lack of cyclin D-CDK4/6 activity prevents the phosphorylation of retinoblastoma protein (Rb), a critical tumor suppressor. This failure to phosphorylate Rb prevents the release of E2F transcription factors, which are essential for the progression from G1 to S phase. This ultimately leads to cell cycle arrest in G1. Similarly, the lack of cyclin B-CDK1 activity prevents the onset of mitosis, causing cell cycle arrest in G2.
Mechanisms Compensating for Cyclin Absence (or attempting to)
While the absence of cyclins severely impairs CDK function, cells have evolved mechanisms to compensate, or at least attempt to mitigate, the effects. These mechanisms are not always effective, however, and often highlight the critical role of cyclins in maintaining cellular homeostasis.
CDK Inhibitors (CKIs) and their Role
CDK inhibitors (CKIs) are a class of proteins that negatively regulate CDK activity. While typically associated with regulation in the presence of cyclins, CKIs can also influence the low basal activity of CDKs in their absence. Some CKIs might actively inhibit the residual activity, preventing potential aberrant phosphorylation events. Others may indirectly influence the stability or degradation of CDKs themselves.
Degradation of CDKs
In the absence of cyclins, CDKs can become unstable and be targeted for degradation by the ubiquitin-proteasome system. This process prevents the accumulation of potentially harmful inactive CDKs, limiting the likelihood of aberrant phosphorylation. The degradation process provides a safety net, minimizing the potential damage caused by dysfunctional CDKs.
Post-translational Modifications
Post-translational modifications (PTMs) of CDKs, such as phosphorylation or acetylation, can also modulate their activity, even in the absence of cyclins. These PTMs can either enhance or suppress the residual activity, depending on the specific modification and the context. The effects of these modifications are not always fully understood and are often context-dependent, making it a complex area of ongoing research.
Clinical Implications and Disease Associations
The disruption of the cyclin-CDK regulatory network has significant clinical implications. Many cancers are characterized by dysregulation of CDKs and cyclins, leading to uncontrolled cell proliferation and tumorigenesis.
Cancer and Dysregulation of Cyclin-CDK Pathways
Mutations in genes encoding CDKs or cyclins are frequently observed in various cancers. These mutations can result in increased CDK activity, even in the absence of appropriate cyclin levels or in the presence of mutated cyclins. This uncontrolled activity contributes to the uncontrolled cell division characteristic of cancerous growths.
Therapeutic Targeting of CDKs
The crucial role of CDKs in cell cycle regulation has made them attractive targets for cancer therapy. CDK inhibitors are currently being developed and used in clinical settings, although their application is complex due to the pleiotropic effects of CDKs on cellular processes. Targeting CDKs effectively requires a deep understanding of their roles in different cell types and cell cycle phases, highlighting the complexity of therapeutic development.
Conclusion: A Tightly Regulated Dance
The relationship between CDKs and cyclins is a tightly choreographed dance, essential for maintaining cellular homeostasis. The consequences of CDK activity in the absence of cyclins underscore the crucial role of cyclins in controlling CDK function. While cells have mechanisms to compensate for the absence of cyclins, these mechanisms are not always effective, highlighting the central importance of cyclin-CDK interactions for proper cell cycle control and overall cellular health. Disruptions in this delicate balance have significant implications for disease, most notably in the development and progression of cancer. Future research focusing on the intricacies of this regulatory system will continue to refine our understanding of cellular control mechanisms and improve therapeutic strategies for various diseases. The study of CDK activity in the absence of cyclins is an ongoing investigation, uncovering nuances in the intricate network regulating cell growth and division, with profound implications for human health.
Latest Posts
Latest Posts
-
Which Of These Is An Optical Medium Of Storage
Apr 01, 2025
-
Approximately Two Thirds Of Indias Gdp Is Made Up Of
Apr 01, 2025
-
Reggie Owns And Operates A Cheese Shop
Apr 01, 2025
-
Intro To Radiologic And Imaging Sciences Chapter 23
Apr 01, 2025
-
What Two Things Does Fair Chase Emphasize
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Happens To Cdks In The Absence Of Cyclins . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
